Chemiluminescence
Chemiluminescence (sometimes "chemoluminescence") is the emission of energy with limited emission of heat (luminescence), as the result of a chemical reaction. Given reactants A and B, with an excited intermediate ◊,
- [A] + [B] → [◊] → [Products] + light
For example, if [A] is luminol and [B] is hydrogen peroxide in the presence of a suitable catalyst we have:
- luminol + H2O2 → 3-APA[◊] → 3-APA + light
where:
- where 3-APA is 3-aminophthalate
- 3-APA[◊] is the excited state fluorescing as it decays to a lower energy level.
The decay of this excited state[◊] to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to Avogadro's number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency.
In a chemical reaction, reactants collide to form a transition state, the enthalpic maximum in a reaction coordinate diagram, which proceeds to the product. Normally, reactants form products of lesser chemical energy. The difference in energy between reactants and products, represented as 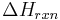 , is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it is rapidly dispersed into the solvent through solvent molecules' rotation and translation. This is how exothermic reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is delivered in an excited electronic state, which then decays into an electronic ground state through either fluorescence or phosphorescence, depending on the spin state of the electronic excited state formed. This is possible because chemical bond formation can occur on a timescale faster than electronic transitions, and therefore can result in discrete products in excited electronic states.
, is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it is rapidly dispersed into the solvent through solvent molecules' rotation and translation. This is how exothermic reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is delivered in an excited electronic state, which then decays into an electronic ground state through either fluorescence or phosphorescence, depending on the spin state of the electronic excited state formed. This is possible because chemical bond formation can occur on a timescale faster than electronic transitions, and therefore can result in discrete products in excited electronic states.
Chemiluminescence differs from fluorescence in that the electronic excited state is derived from the product of a chemical reaction rather than the more typical way of creating electronic excited states, namely absorption. It is the antithesis of a photochemical reaction, in which light is used to drive an endothermic chemical reaction. Here, light is generated from a chemically exothermic reaction.
A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence.
Contents |
Liquid-phase reactions
- Luminol in an alkaline solution with hydrogen peroxide in the presence of iron or copper,[1] or an auxiliary oxidant,[2] produces chemiluminescence. The luminol reaction is
- luminol + H2O2 → 3-APA[◊] → 3-APA + light
Gas-phase reactions
- One of the oldest known chemoluminescent reactions is that of elemental white phosphorus oxidizing in moist air, producing a green glow. This is a gas-phase reaction of phosphorus vapor, above the solid, with oxygen producing the excited states (PO)2 and HPO.[3]
- Another gas phase reaction is the basis of nitric oxide detection in commercial analytic instruments applied to environmental air-quality testing. Ozone is combined with nitric oxide to form nitrogen dioxide in an activated state.
- NO+O3 → NO2[◊]+ O2
- The activated NO2[◊] luminesces broadband visible to infrared light as it reverts to a lower energy state. A photomultiplier and associated electronics counts the photons that are proportional to the amount of NO present. To determine the amount of nitrogen dioxide, NO2, in a sample (containing no NO) it must first be converted to nitric oxide, NO, by passing the sample through a converter before the above ozone activation reaction is applied. The ozone reaction produces a photon count proportional to NO that is proportional to NO2 before it was converted to NO. In the case of a mixed sample that contains both NO and NO2, the above reaction yields the amount of NO and NO2 combined in the air sample, assuming that the sample is passed through the converter. If the mixed sample is not passed through the converter, the ozone reaction produces activated NO2[◊] only in proportion to the NO in the sample. The NO2 in the sample is not activated by the ozone reaction. Though unactivated NO2 is present with the activated NO2[◊], photons are emitted only by the activated species that is proportional to original NO. Final step: Subtract NO from (NO + NO2) to yield NO2[4]
Enhanced chemiluminescence
Enhanced chemiluminescence is a common technique for a variety of detection assays in biology. A horseradish peroxidase enzyme (HRP) is tethered to the molecule of interest (usually through labeling an immunoglobulin that specifically recognizes the molecule). This enzyme complex, then catalyzes the conversion of the enhanced chemiluminescent substrate into a sensitized reagent in the vicinity of the molecule of interest, which on further oxidation by hydrogen peroxide, produces a triplet (excited) carbonyl, which emits light when it decays to the singlet carbonyl. Enhanced chemiluminescence allows detection of minute quantities of a biomolecule. Proteins can be detected down to femtomole quantities,[5] well below the detection limit for most assay systems.
Applications
- Gas analysis: for determining small amounts of impurities or poisons in air. Other compounds can also be determined by this method (ozone, N-oxides, S-compounds). A typical example is NO determination with detection limits down to 1 ppb
- Analysis of inorganic species in liquid phase
- Analysis of organic species: useful with enzymes, where the substrate is not directly involved in chemiluminescence reaction, but the product is
- Detection and assay of biomolecules in systems such as ELISA and Western blots
- DNA sequencing using pyrosequencing
- Lighting objects. Chemiluminescence kites,[6] emergency lighting, glow sticks (party decorations).
- Combustion analysis: Certain radical species (such as CH* and OH*) give off radiation at specific wavelengths. The heat release rate is calculated by measuring the amount of light radiated from a flame at those wavelengths.
- Children's toys
See also
References
- ^ "Luminol chemistry laboratory demonstration". http://www.3rd1000.com/labs/lumine.htm. Retrieved 2006-03-29.
- ^ "Investigating luminol" (PDF). Salters Advanced Chemistry. http://web.archive.org/web/20040920053056/http://www.york.ac.uk/org/seg/salters/chemistry/ResourceSheets/luminol.PDF. Retrieved 2006-03-29.
- ^ Rauhut, Michael M. (1985), Chemiluminescence. In Grayson, Martin (Ed) (1985). Kirk-Othmer Concise Encyclopedia of Chemical Technology (3rd ed), pp 247 John Wiley and Sons. ISBN 0-471-51700-3
- ^ Air Zoom | Glowing with Pride. Fannation.com. Retrieved on 2011-11-22.
- ^ Enhanced CL review. Biocompare.com (2007-06-04). Retrieved on 2011-11-22.
- ^ Kinn, John J "Chemiluminescent kite" U.S. Patent 4,715,564issued 12/29/1987
|
|||||||||||||||||||||||