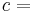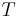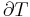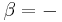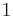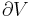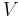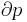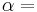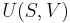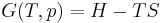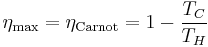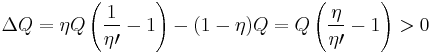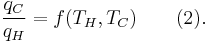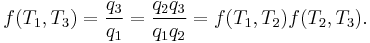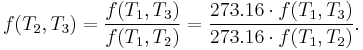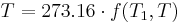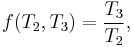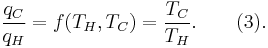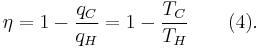Carnot's theorem (thermodynamics)
Carnot's theorem, developed in 1824, also called Carnot's rule is a principle that specifies limits on the maximum efficiency any heat engine can obtain, which thus solely depends on the difference between the hot and cold temperature reservoirs.
Carnot's theorem states:
- All irreversible heat engines between two heat reservoirs are less efficient than a Carnot engine operating between the same reservoirs.
- All reversible heat engines between two heat reservoirs are equally efficient with a Carnot engine operating between the same reservoirs.
The formula for this maximum efficiency is
where TC is the absolute temperature of the cold reservoir, TH is the absolute temperature of the hot reservoir, and the efficiency 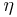 is the ratio of the work done by the engine to the heat drawn out of the hot reservoir.
is the ratio of the work done by the engine to the heat drawn out of the hot reservoir.
Based on modern thermodynamics, Carnot's theorem is a result of the second law of thermodynamics. Historically, however, it was based on contemporary caloric theory and preceded the establishment of the second law.[1]
Contents |
Proof
The theorem may be proved in the following way for the cases of the irreversible and the reversible heat engines.[2]
Irreversible engine
The derivation assumes an irreversible heat engine that has an efficiency 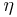 , operating between reservoirs
, operating between reservoirs 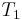 and
and 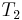 . It is combined with a reversed Carnot engine that has efficiency
. It is combined with a reversed Carnot engine that has efficiency 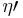 as shown by the diagram to the right.
as shown by the diagram to the right.
If 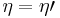 , then the combined engine does nothing in a cycle, which contradicts the irreversibility.
, then the combined engine does nothing in a cycle, which contradicts the irreversibility.
If 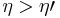 , then the net effect of the combined engine is draining heat
, then the net effect of the combined engine is draining heat
from the colder reservoir 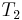 and releases the same amount to the hotter reservoir
and releases the same amount to the hotter reservoir 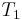 without affecting any other changes. This is clearly in violation of the Clausius statement of the second law.
without affecting any other changes. This is clearly in violation of the Clausius statement of the second law.
The conclusion is that the only possibility remaining is 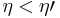 .
.
Reversible engine
In the case of a reversible heat engine, the proof may be conducted similarly, in that 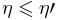 .
.
The said engine is reversed and combined with a regular Carnot engine. In a similar manner the conclusion is obtained: 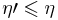 . Thus,
. Thus,
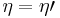 .
.
Definition of thermodynamic temperature
The efficiency of the engine is the work divided by the heat introduced to the system or
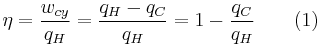 ,
,
where wcy is the work done per cycle. Thus, the efficiency depends only on qC/qH.
Because all reversible engines operating between the same heat reservoirs are equally efficient, any reversible heat engine operating between temperatures T1 and T2 must have the same efficiency, meaning, the efficiency is the function of the temperatures only:
In addition, a reversible heat engine operating between temperatures T1 and T3 must have the same efficiency as one consisting of two cycles, one between T1 and another (intermediate) temperature T2, and the second between T2andT3. This can only be the case if
Specializing to the case that 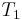 is a fixed reference temperature: the temperature of the triple point of water. Then for anyT2and T3,
is a fixed reference temperature: the temperature of the triple point of water. Then for anyT2and T3,
Therefore, if thermodynamic temperature is defined by
then the function f, viewed as a function of thermodynamic temperature, is
and the reference temperature T1 has the value 273.16. (Of course any reference temperature and any positive numerical value could be used—the choice here corresponds to the Kelvin scale.)
It follows immediately that
Substituting Equation 3 back into Equation 1 gives a relationship for the efficiency in terms of temperature:
Applicability to fuel cells and batteries
Since fuel cells and batteries can generate useful power when all components of the system are at the same temperature (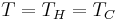 ), they are clearly not limited by Carnot's theorem, which states that no power can be generated when
), they are clearly not limited by Carnot's theorem, which states that no power can be generated when 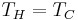 . This is because Carnot's theorem applies to engines converting thermal energy to work, whereas fuel cells and batteries instead convert chemical energy to work.[3] Nevertheless, the second law of thermodynamics still provides restrictions on fuel cell and battery energy conversion.[4]
. This is because Carnot's theorem applies to engines converting thermal energy to work, whereas fuel cells and batteries instead convert chemical energy to work.[3] Nevertheless, the second law of thermodynamics still provides restrictions on fuel cell and battery energy conversion.[4]
References
- ^ John Murrell. "A Very Brief History of Thermodynamics". http://www.sussex.ac.uk/chemistry/documents/a_thermodynamics_history.pdf. Retrieved October 5,2010.
- ^ "Lecture 10: Carnot theorem". Feb 7,2005. http://seit.unsw.adfa.edu.au/staff/sites/hrp/Literature/articles/CarnotTheorem.pdf. Retrieved October 5,2010.
- ^ "Fuel Cell versus Carnot Efficiency". http://docs.google.com/viewer?a=v&q=cache:KkaC5XqVzVgJ:filer.case.edu/org/hsfcproject/Unit%252036/36.1.3%2520Fuel%2520Cell%2520versus%2520Carnot%2520Efficiency.ppt+%22carnot+limit%22+%22fuel+cell%22&hl=en&gl=us&pid=bl&srcid=ADGEESj0jJDxV7N40e6SinnUfC9X_wqU6GHB4aPX6BlFRSYsfeixt1hGvyGLMAthvrl_Ws6k4r-Z8AdtYkjDqhVrIUwjO6rSA8y5JnGnqt73LWITIpc3KdeTUBe24GkaZcLs1BpHUxh5&sig=AHIEtbROSMlotD3mw6tegO0_v-uhvVwI2g. Retrieved Feb 20, 2011.
- ^ "Fuel cell efficiency redefined : Carnot limit reassessed". http://cat.inist.fr/?aModele=afficheN&cpsidt=17183054. Retrieved Feb 20, 2011.