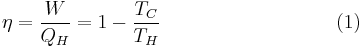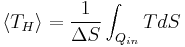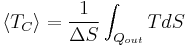Carnot heat engine
A Carnot heat engine[2] is a hypothetical engine that operates on the reversible Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded upon by Benoît Paul Émile Clapeyron in 1834 and mathematically elaborated upon by Rudolf Clausius in the 1850s and 60s from which the concept of entropy emerged.
Every thermodynamic system exists in a particular state. A thermodynamic cycle occurs when a system is taken through a series of different states, and finally returned to its initial state. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine.
A heat engine acts by transferring energy from a warm region to a cool region of space and, in the process, converting some of that energy to mechanical work. The cycle may also be reversed. The system may be worked upon by an external force, and in the process, it can transfer thermal energy from a cooler system to a warmer one, thereby acting as a refrigerator or heat pump rather than a heat engine.
In the adjacent diagram, from Carnot's 1824 work, Reflections on the Motive Power of Fire,[3] there are "two bodies A and B, kept each at a constant temperature, that of A being higher than that of B. These two bodies, to which we can give or from which we can remove the heat without causing their temperatures to vary, exercise the functions of two unlimited reservoirs of caloric. We will call the first the furnace and the second the refrigerator.”[4] Carnot then explains how we can obtain motive power, i.e. “work”, by carrying a certain quantity of heat from body A to body B.
Contents |
Modern diagram
The previous image shows the original piston-and-cylinder diagram used by Carnot in discussing his ideal engine. The figure at right shows a block diagram of a generic heat engine, such as the Carnot engine. In the diagram, the “working body” (system), a term introduced by Clausius in 1850, can be any fluid or vapor body through which heat Q can be introduced or transmitted to produce work. Carnot had postulated that the fluid body could be any substance capable of expansion, such as vapor of water, vapor of alcohol, vapor of mercury, a permanent gas, or air, etc. Although, in these early years, engines came in a number of configurations, typically QH was supplied by a boiler, wherein water was boiled over a furnace; QC was typically a stream of cold flowing water in the form of a condenser located on a separate part of the engine. The output work W here is the movement of the piston as it is used to turn a crank-arm, which was then typically used to turn a pulley so to lift water out of flooded salt mines. Carnot defined work as “weight lifted through a height”.
Carnot's theorem
Carnot's theorem is a formal statement of this fact: No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between the same reservoirs.
This maximum efficiency 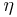 is defined to be:
is defined to be:
where
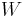 is the work done by the system (energy exiting the system as work),
is the work done by the system (energy exiting the system as work),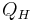 is the heat put into the system (heat energy entering the system),
is the heat put into the system (heat energy entering the system),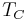 is the absolute temperature of the cold reservoir, and
is the absolute temperature of the cold reservoir, and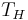 is the absolute temperature of the hot reservoir.
is the absolute temperature of the hot reservoir.
A corollary to Carnot's theorem states that: All reversible engines operating between the same heat reservoirs are equally efficient.
In other words, maximum efficiency is achieved if and only if no new entropy is created in the cycle. Otherwise, since entropy is a state function, the required dumping of heat into the environment to dispose of excess entropy leads to a reduction in efficiency. So Equation (1) gives the efficiency of any reversible heat engine.
The Coefficient of Performance (COP) of the heat engine is the reciprocal of its efficiency.
Efficiency of real heat engines
Carnot realized that in reality it is not possible to build a thermodynamically reversible engine, so real heat engines are less efficient than indicated by Equation (1). Nevertheless, Equation (1) is extremely useful for determining the maximum efficiency that could ever be expected for a given set of thermal reservoirs.
Although Carnot's cycle is an idealisation, the expression of Carnot efficiency is still useful. Consider the average temperatures,
at which heat is input and output, respectively. Replace TH and TC in Equation (1) by <TH> and <TC> respectively.
For the Carnot cycle, or an equivalent, <TH> is the highest temperature available and <TC> the lowest. For other less efficient cycles, <TH> will be lower than TH, and <TC> will be higher than TC. This can help illustrate, for example, why a reheater or a regenerator can improve thermal efficiency.
- See also: Heat engine (efficiency and other performance criteria)
See also
- Carnot cycle
- Heat engine
- Thermal efficiency
- History of the internal combustion engine
- Adiabatic process
References
- ^ Figure 1 in Carnot (1824, p. 17) and Carnot (1890, p. 63). In the diagram, the diameter of the vessel is large enough to bridge the space between the two bodies, but in the model, the vessel is never in contact with both bodies simultaneously. Also, the diagram shows an unlabeled axial rod attached to the outside of the piston.
- ^ In French, Carnot uses machine à feu, which Thurston translates as heat-engine or steam-engine. In a footnote, Carnot distinguishes the steam-engine (machine à vapeur) from the heat-engine in general. (Carnot, 1824, p. 5 and Carnot, 1890, p. 43)
- ^ Sometimes translated as Reflections on the Motive Power of Fire
- ^ English translation by Thurston (Carnot, 1890, p. 51-52).
- Carnot, Sadi (1824). Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance. Paris: Bachelier. http://books.google.com/books?id=YcY9AAAAMAAJ. (French)
- Carnot, Sadi; Thurston, Robert Henry (editor and translator) (1890). Reflections on the Motive Power of Heat and on Machines Fitted to Develop That Power. New York: J. Wiley & Sons. (full text of 1897 ed.)) (html)
- Feynman, Richard P.; Leighton, Robert B.; Sands, Matthew (1963). The Feynman Lectures on Physics. Addison-Wesley Publishing Company. pp. 44–4f. ISBN 0201021161.
- Halliday, David; Resnick, Robert (1978). Physics (3rd ed. ed.). John Wiley & Sons. pp. 541–548. ISBN 0471024562.
- Kittel, Charles; Kroemer, Herbert (1980). Thermal Physics (2nd ed. ed.). W. H. Freeman Company. ISBN 0-7167-1088-9.
|
||||||||||||||