Autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature at which it will spontaneously ignite in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to supply the activation energy needed for combustion. The temperature at which a chemical will ignite decreases as the pressure increases or oxygen concentration increases. It is usually applied to a combustible fuel mixture.
Autoignition temperatures of liquid chemicals are typically measured using a 500 mL flask placed in a temperature controlled oven in accordance with the procedure described in ASTM E659.[1]
Contents |
Autoignition equation
The time 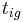 it takes for a material to reach its autoignition temperature
it takes for a material to reach its autoignition temperature 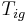 when exposed to a heat flux
when exposed to a heat flux 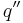 is given by the following equation
is given by the following equation
where k = thermal conductivity (W/(m·K)), ρ = density (kg/m³), and c = specific heat capacity (J/(kg·K)) of the material of interest. 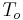 is the temperature, in kelvins, the material starts at (or the temperature of the bulk material), and
is the temperature, in kelvins, the material starts at (or the temperature of the bulk material), and 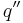 is the heat flux (W/m²) incident to the material.
is the heat flux (W/m²) incident to the material.
To be consistent in units the group ![\left[ \frac{T_{ig}-T_{o}}{q''} \right]](/2012-wikipedia_en_all_nopic_01_2012/I/1436167927cf64474ad747ab12f6097c.png) should be squared.
should be squared.
Autoignition point of selected substances
Temperatures vary widely in the literature and should only be used as estimates. Factors which may cause variation include partial pressure of oxygen, altitude, humidity, and amount of time required for ignition.
- Triethylborane: −20 °C (−4 °F)
- Silane: <21 °C (70 °F)
- White phosphorus: 34 °C (93 °F)
- Carbon disulfide: 90 °C (194 °F)
- Diethyl ether: 160 °C (320 °F)[3]
- Diesel or Jet A-1: 210 °C (410 °F)
- Gasoline (Petrol): 246–280 °C (475–536 °F)[4]
- Butane: 405 °C (761 °F)[5]
- Paper: 450 °C (842 °F)[6] or 218°-246°C (424-474°F)[7]
- Magnesium: 473 °C (883 °F)
- Hydrogen: 536 °C (997 °F)[8]
For paper, there is considerable variation between sources. Part of this is because it takes longer for combustion to start at lower temperatures.[9]
See also
- Pyrolysis
- Flash Point
- Gas burner (For flame temperatures, combustion heat energy values and ignition temperatures)
References
- ^ E659 – 78 (Reapproved 2000), "Standard Test Method for Autoignition Temperature of Liquid Chemicals", ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959
- ^ Principles of Fire Behavior. ISBN 0-8273-7732-0. 1998.
- ^ "Diethyl Ether - Safety Properties". http://www.wolframalpha.com/input/?i=diethyl+ether. by Wolfram|Alpha curated data, 2009; Wolfram Mathematica ChemicalData
- ^ "Fuels and Chemicals - Auto Ignition Temperatures". http://www.engineeringtoolbox.com/fuels-ignition-temperatures-d_171.html. depending on grade
- ^ "Butane - Safety Properties". http://www.wolframalpha.com/input/?i=butane. by Wolfram|Alpha curated data, 2009; Wolfram Mathematica ChemicalData
- ^ Jens Borch, Richard E. Mark, M. Bruce Lyne. Handbook of Physical Testing of Paper. http://books.google.com/books?id=qa-I8QAOUL8C&pg=PA406&lpg=PA406&dq=flash+point+of+paper&ct=result#PPA406,M1.
- ^ Tony Cafe. "Physical Constants for Investigators". Journal of Australian Fire Investigators. http://www.tcforensic.com.au/docs/article10.html.
- ^ "Hydrogen - Safety Properties". http://www.wolframalpha.com/input/?i=hydrogen&a=*C.hydrogen-_*Chemical-. by Wolfram|Alpha curated data, 2009; Wolfram Mathematica ChemicalData
- ^ Forest Products Laboratory (1964). "IGNITION AND CHARRING TEMPERATURES OF WOOD". Forest Service U. S. Department of Agriculture. http://www.fpl.fs.fed.us/documnts/fplmisc/rpt1464.pdf.
External links
|
|||||||||||||||||||||||
![t_{ig} = \left ( \frac{\pi}{4} \right ) \left (k \rho c \right )\left [ \frac{T_{ig}-T_{o}}{q''} \right]^2](/2012-wikipedia_en_all_nopic_01_2012/I/1968b7d5a90781ae54f8f0566d0af074.png)