Coulomb
|
|
|
| Unit system: | SI derived unit |
| Unit of... | Electric charge |
| Symbol: | C |
| Named after: | Charles-Augustin de Coulomb |
|
|
|
| 1 C in... | is equal to... |
| SI base units | 1 A s |
| CGS units | 2997924580 statC |
| Atomic units | 6.24150965(16)×1018 e[1] |
The coulomb (symbol: C) is the SI derived unit of electric charge. It is defined as the charge transported by a steady current of one ampere in one second:
One coulomb is also the amount of excess charge on the positive side of a capacitance of one farad charged to a potential difference of one volt:
Contents |
Name and notation
This SI unit is named after Charles-Augustin de Coulomb. As with every SI unit whose name is derived from the proper name of a person, the first letter of its symbol is upper case (C). When an SI unit is spelled out in English, it should always begin with a lower case letter (coulomb), except where any word would be capitalized, such as at the beginning of a sentence or in capitalized material such as a title. Note that "degree Celsius" conforms to this rule because the "d" is lowercase. —Based on The International System of Units, section 5.2.[2]
Definition
In the SI system, the coulomb is defined in terms of the ampere and second: 1C = 1A × 1s.[3] The second is defined in terms of a frequency which is naturally emitted by caesium atoms.[4] The ampere is defined using Ampère's force law;[5] the definition relies in part on the mass of the international prototype kilogram, a metal cylinder housed in France.[6] In practice, the watt balance is used to measure amperes with the highest possible accuracy.[6]
SI prefixes
| Submultiples | Multiples | |||||
|---|---|---|---|---|---|---|
| Value | Symbol | Name | Value | Symbol | Name | |
| 10−1 C | dC | decicoulomb | 101 C | daC | decacoulomb | |
| 10−2 C | cC | centicoulomb | 102 C | hC | hectocoulomb | |
| 10−3 C | mC | millicoulomb | 103 C | kC | kilocoulomb | |
| 10−6 C | µC | microcoulomb | 106 C | MC | megacoulomb | |
| 10−9 C | nC | nanocoulomb | 109 C | GC | gigacoulomb | |
| 10−12 C | pC | picocoulomb | 1012 C | TC | teracoulomb | |
| 10−15 C | fC | femtocoulomb | 1015 C | PC | petacoulomb | |
| 10−18 C | aC | attocoulomb | 1018 C | EC | exacoulomb | |
| 10−21 C | zC | not used | 1021 C | ZC | zettacoulomb | |
| 10−24 C | yC | not used | 1024 C | YC | yottacoulomb | |
| Common multiples are in bold face. | ||||||
See also SI prefix.
Conversions
- The magnitude of the electrical charge of one mole of elementary charges (approximately 6.022×1023, or Avogadro's number) is known as a faraday unit of charge (closely related to the Faraday constant). One faraday is equal to 96485.3399 coulombs. In terms of Avogadro's number (NA), one coulomb is equal to approximately 1.036 × NA ×10−5 elementary charges.
- one ampere-hour = 3600 C, 1 mAh = 3.6 C
- The elementary charge is 1.602176487×10−19 C[1]
- One statcoulomb (statC), the CGS electrostatic unit of charge (esu), is approximately 3.3356×10−10 C or about 1/3 nC.
- One coulomb is the magnitude (absolute value) of electrical charge in 6.24150965(16)×1018 protons or electrons. [1]
Relation to elementary charge
The elementary charge, the charge of a proton (equivalently, the negative of the charge of an electron), is approximately 1.602176487(40)×10−19 C.[1] In SI, the elementary charge in coulombs is an approximate value: no experiment can be infinitely accurate. However, in other unit systems, the elementary charge has an exact value by definition, and other charges are ultimately measured relative to the elementary charge.[7] For example, in conventional electrical units, the values of the Josephson constant KJ and von Klitzing constant RK are exact defined values (written KJ-90 and RK-90), and it follows that the elementary charge 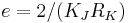 is also an exact defined value in this unit system.[7] Specifically,
is also an exact defined value in this unit system.[7] Specifically, 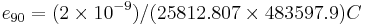 exactly.[7] SI itself may someday change its definitions in a similar way.[7] For example, one possible proposed redefinition is "the ampere...is [defined] such that the value of the elementary charge e (charge on a proton) is exactly 1.602176487×10−19 coulomb"[8] This proposal is not yet accepted as part of the SI system: The SI definitions are unlikely to change until at least 2015.[9]
exactly.[7] SI itself may someday change its definitions in a similar way.[7] For example, one possible proposed redefinition is "the ampere...is [defined] such that the value of the elementary charge e (charge on a proton) is exactly 1.602176487×10−19 coulomb"[8] This proposal is not yet accepted as part of the SI system: The SI definitions are unlikely to change until at least 2015.[9]
In everyday terms
- The charges in static electricity from rubbing materials together are typically a few microcoulombs.[10]
- The amount of charge that travels through a lightning bolt is typically around 15 C, although large bolts can be up to 350 C.[11]
- The amount of charge that travels through a typical alkaline AA battery is about 5 kC = 5000 C = 1400 mAh. After that charge has flowed, the battery must be discarded or recharged.[12]
- According to Coulomb's Law, two point charges of +1 C, placed one meter apart, would experience a repulsive force of 9×109 N, a force roughly equal to the weight of 920,000 metric tons of mass on the surface of the Earth.
See also
- Abcoulomb, a cgs unit of charge
- Ampère's circuital law
- Coulomb's law
- Electrostatics
- Elementary charge
- Faraday (unit), an obsolete unit
- Quantity of electricity
References
- ^ a b c d Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). "CODATA Recommended Values of the Fundamental Physical Constants: 2006". Rev. Mod. Phys. 80: 633–730. Bibcode 2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633. http://physics.nist.gov/cuu/Constants/codata.pdf. Direct link to value. The inverse value (the number of elementary charges in 1C) is given by 1/[1.602176487(40)×10-19] = 6.24150965(16)×1018.
- ^ "SI Brochure, Appendix 1,". BIPM. p. 144. http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf.
- ^ "SI brochure, section 2.2.2". BIPM. http://www.bipm.org/en/si/si_brochure/chapter2/2-2/table3.html.
- ^ "SI brochure, section 2.2.1.3". BIPM. http://www.bipm.org/en/si/si_brochure/chapter2/2-1/second.html.
- ^ "SI brochure, section 2.2.1.4". BIPM. http://www.bipm.org/en/si/si_brochure/chapter2/2-1/ampere.html.
- ^ a b "Watt Balance". BIPM. http://www.bipm.org/en/scientific/elec/watt_balance/.
- ^ a b c d Mills, I. M.; Mohr, P. J.; Quinn, T. J.; Taylor, B. N.; Williams, E. R. (2005). "Redefinition of the kilogram: a decision whose time has come". Metrologia 42 (2): 71. Bibcode 2005Metro..42...71M. doi:10.1088/0026-1394/42/2/001.
- ^ Report of the CCU to the 23rd CGPM
- ^ Anon (November 2010). "BIPM Bulletin". BIPM. http://www.bipm.org/utils/en/pdf/BIPM_Bulletin.pdf. Retrieved 2011-01-28.
- ^ Martin Karl W. Pohl. "Physics: Principles with Applications". DESY. http://www-zeuthen.desy.de/~pohlmadq/teach/112/ch16.pdf.
- ^ Hasbrouck, Richard. Mitigating Lightning Hazards, Science & Technology Review May 1996. Retrieved on 2009-04-26.
- ^ How to do everything with digital photography – David Huss at Google Books, "The capacity range of an AA battery is typically from 1100–2200 mAh."
|
||||||||||||||||||||