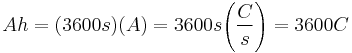Ampere-hour
An ampere-hour or amp-hour (symbol Ah , A·h, A h) is a unit of electric charge, with sub-units milliampere-hour (mAh) and milliampere second (mAs). One ampere-hour is equal to 3,600 coulombs (ampere-seconds), the electric charge transferred by a steady current of one ampere for one hour.[1] The ampere-hour is frequently used in measurements of electrochemical systems such as electroplating and electrical batteries. The commonly seen milliampere-hour (mAh or mA·h) is one-thousandth of an ampere-hour (3.6 coulombs).
A milliampere second (mAs or mA·s) is a unit of measure used in X-ray diagnostic imaging and radiation therapy. This quantity is proportional to the total X-ray energy produced by a given X-ray tube operated at a particular voltage.[2] The same total dose can be delivered in different time periods depending on the X-ray tube current.
The Faraday constant is the charge on one mole of electrons; approximately equal to 26.8 ampere-hours. It is used in electrochemical calculations.
An ampere-hour is not a unit of energy. In a battery system, for example, accurate calculation of the energy delivered requires integration of the power delivered (product of instantaneous voltage and instantaneous current) over the discharge interval. Generally, the battery voltage varies during discharge; an average value may be used to approximate the integration of power. [3]
In summary, the higher the mAh, the longer the battery will last.
References
- ^ "Full Conversion Table (sorted by Category)" Allmeasures.com, 2004, webpage: AM-Conversion-table.
- ^ X-ray Safety Handbook, 9.0 Terms and Definitions, VirginiaTech Environmental, Health and Safety Services
- ^ National Research Council (U.S.), Meeting the energy needs of future warriors National Academies Press, 2004 ISBN 0309092612 page 27