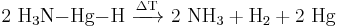Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.
The origin of the word amalgam is from the Medieval Latin amalgama, "alloy of mercury (esp. with gold or silver)," perhaps an alteration of L. malagma "poultice, plaster," probably from Arabic al-malgham "an emollient poultice or unguent for sores (especially warm)", perhaps from Greek malagma "softening substance," from malassein "to soften" from malakos "soft".[1]
Contents |
Known amalgams and their uses
Dental amalgam
Dentistry has used alloys of mercury with metals such as silver, copper, indium, tin and zinc. Amalgam is an "excellent and versatile restorative material" and is used in dentistry for a number of reasons. It is inexpensive and relatively easy to use and manipulate during placement; it remains soft for a short time so it can be packed to fill any irregular volume, and then forms a hard compound. Amalgam possesses greater longevity when compared to other direct restorative materials, such as composite. However, this difference has decreased with continual development of composite resins.
Amalgam is typically compared to resin-based composites because many applications are similar and many physical properties and costs are comparable.
Potassium amalgams
For the alkali metals, amalgamation is exothermic, and distinct chemical forms can be identified, such as KHg and KHg2.[2] KHg is a gold-coloured compound with a melting point of 178 °C, and KHg2 a silver-coloured compound with a melting point of 278 °C. These amalgams are very sensitive to air and water, but can be worked with under dry nitrogen. The Hg-Hg distance is around 300 picometres, Hg-K around 358 pm.[2]
Phases K5Hg7 and KHg11 are also known; rubidium, strontium and barium undecamercurides are known and isostructural. Sodium amalgam (NaHg2) has a different structure, with the mercury atoms forming hexagonal layers, and the sodium atoms a linear chain which fits into the holes in the hexagonal layers, but the potassium atom is too large for this structure to work in KHg2.
Sodium amalgam
Sodium amalgam is produced as a by product of the chloralkali process and used as an important reducing agent in organic and inorganic chemistry. With water it decomposes into concentrated sodium hydroxide solution, hydrogen and mercury, which can then return to the chloralkali process anew. If absolutely water-free alcohol is used instead of water, an alkoxide of sodium is produced instead of the alkali solution.
Ammonium amalgam
Discovered in 1808 by both Humphry Davy and Jöns Jakob Berzelius.
It is grey, soft, spongy mass which decomposes readily at room temperature or in contact with water or alcohol:
It is highly toxic and harmful to the environment. Its CAS number is 26497-91-6.
Use in mining
Mercury has been used in the gold and silver mining methods because of the convenience and easiness with which mercury will amalgamate with them. In gold placer mining, in which minute specks of gold are washed from sand or gravel deposits, mercury was often used to separate the gold from other heavy minerals.
After all of the practical metal had been taken out from the ore, the mercury was dispensed down a long copper trough, which formed a thin coating of mercury on the exterior. The waste ore was then transferred down the trough, and any gold in the waste that was amalgamated with the mercury. This coating would sometimes get scraped off and refined to get rid of the mercury, leaving behind somewhat high purity gold.
Mercury amalgamation was first useful to silver ores with the development of the patio process in Mexico in 1557. There were also additional amalgamation processes that were created for processing silver ores, including pan amalgamation and the Washoe process.
Gold amalgam
Produced when mercury is used for gold extraction.
Gold extraction (mining)
This has proved effective where gold fines ("flour gold") would not be extractable from ore using hydro-mechanical methods. Large amounts of mercury were used in placer mining, where deposits composed largely of decomposed granite slurry were separated in long runs of "riffle boxes", with mercury dumped in at the head of the run. The amalgam formed is a heavy solid mass of dull gray color. (The use of mercury in 19th century placer mining in California, now prohibited, has caused extensive pollution problems in riverine and estuarine environments, ongoing to this day.) Sometimes substantial slugs of amalgam are found in downstream river and creek bottoms by amateur wet-suited miners seeking gold nuggets with the aid of an engine-powered water vacuum mounted on a float.
Gold extraction (ore processing)
Where stamp mills were used to crush gold-bearing ore to fines, a part of the extraction process involved the use of mercury-wetted copper plates, over which the crushed fines were washed. A periodic scraping and re-mercurizing of the palate resulted in amalgam for further processing.
Gold extraction (retorting)
Amalgam obtained by either process was then heated in a distillation retort, recovering the mercury for reuse and leaving behind the gold. As this released mercury vapors to the atmosphere, the process could induce adverse health effects and long term pollution.
Today, mercury amalgamation has been replaced by other methods to recuperate gold and silver from ore in developed nations. Hazards of mercurial toxic waste have played a major role in the phasing out of the mercury amalgamation processes. However mercury amalgamation is still regularly used by small-scale gold placer miners (often illegally) & particularly in developing countries.
Aluminium amalgam
Aluminium amalgam is used as a reducing agent.
Thallium amalgam
Thallium amalgam has a freezing point of −58 °C, lower than mercury's, and so has found a use in low temperature thermometers.
Tin amalgam
Tin amalgam was used in the middle of the 19th century as a reflective mirror coating.[3]
Amalgam probe
Mercury salts are, compared to mercury metal and amalgam, highly toxic due to their solubility in water. The presence of these salts in water can be detected with a probe that uses the readiness of mercury ions to form an amalgam with copper. A nitric acid solution of salts under investigation is applied to a piece of copper foil and any mercury ions present will leave spots of silvery-coloured amalgam. Silver ions leave similar spots but are easily washed away, making this a means of distinguishing silver from mercury.
The redox reaction involved where mercury oxidizes the copper is:
- Hg2+ + Cu → Hg + Cu2+
See also
References
- ^ http://www.etymonline.com/index.php?term=amalgam
- ^ a b E J Duwell; N C Baenziger (1955). "The Crystal Structures of KHg and KHg2". Acta Cryst. 8 (11): 705–710. doi:10.1107/S0365110X55002168.
- ^ "Die Sendung mit der Maus, Sachgeschichte vom Spiegel" (in German). http://leifi.physik.uni-muenchen.de/web_ph09/umwelt_technik/10spiegelbau_maus/spegelbau.htm. Retrieved 2009-04-24
Further reading
- Prandtl, W.: Humphry Davy, Jöns Jacob Berzelius, zwei führende Chemiker aus der ersten Hälfte des 19. Jahrhunderts. Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1948
- Hofmann, H., Jander, G.: Qualitative Analyse, 1972, Walter de Gruyter, ISBN 3110036533