Allene
An allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are called cumulenes. Allenes are much more reactive than most other alkenes. For example, their reactivity with gaseous chlorine is more like the reactivity of alkynes.
Contents |
Structure and bonding
Geometry
The central carbon of allene forms two sigma bonds and two pi bonds. The central carbon is sp-hybridized, and the two terminal carbons are sp2-hybridized. The bond angle formed by the three carbons is 180°, indicating linear geometry for the carbons of allene.
Symmetry
The symmetry and isomerism of allenes has long fascinated organic chemists.[1] For allenes with four identical substituents, there exist two twofold axes of rotation through the center carbon, inclined at 45° to the CH2 planes at either end of the molecule. The molecule can thus be thought of as a two-bladed propeller. A third twofold axis of rotation passes through the C=C=C bonds, and there is a mirror plane passing through both CH2 planes. Thus this class of molecules belong to the D2d point group. Because of the symmetry, an unsubstituted allene has no net dipole moment.
An allene with two different substituents on each of the two carbons will be chiral because there will no longer be any mirror planes. Where A has a greater priority than B according to the Cahn-Ingold-Prelog priority rule, the configuration of the axial chirality can be determined by considering the top, then the bottom. For the bottom, only the group of higher priority need be considered.
Synthesis
Although allenes often require specialized syntheses, the parent, propadiene is produced on a large scale as an equilibrium mixture with methylacetylene:
- H2C=C=CH2
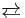 CH3C≡CH
CH3C≡CH
This mixture, known as MAPP gas, is commercially available.
Laboratory methods for the formation of allenes include:
- from geminal dihalocyclopropanes and organolithium compounds in the Skattebøl rearrangement.
- from reaction of certain terminal alkynes with formaldehyde copper(II) bromide and added base[2]
- from dehydrohalogenation of certain dihalides.[3]
- from reaction of a triphenylphosphinyl ester with an acid halide, a Wittig reaction accompanied by dehydrohalogenation[4]
See also
- Compounds with three or more adjacent carbon-carbon double bonds are called cumulenes.
- The allene motif is frequently encountered in carbomers.
- Using a suitable catalyst (e.g. Wilkinson's catalyst), it is possible to reduce just one of the double bonds of an allene.[5]
References
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7, http://books.google.com/books?id=JDR-nZpojeEC&printsec=frontcover
- ^ Organic Syntheses, Coll. Vol. 7, p.276 (1990); Vol. 63, p.203 (1985). Link
- ^ Organic Syntheses, Coll. Vol. 5, p.22 (1973); Vol. 42, p.12 (1962) Link
- ^ Helv. Chim. Acta, 63, 438 (1980); Org. Synth. Coll., 7, 232 (1990)
- ^ Bhagwat; Devaprabhakara (1972). "Selective hydrogenation of allenes with chlorotris-(triphenylphosphine) rhodium catalyst". Tetrahedron Lett. 13 (15): 1391. doi:10.1016/S0040-4039(01)84636-0.
Further reading
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "allenes".
- Allene chemistry Kay M. Brummond (Editor) Thematic Series in the Open Access Beilstein Journal of Organic Chemistry