Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic but tend to be more reactive.
Contents |
Chemical properties
Alkynes are characteristically more unsaturated than alkenes. Thus they add two equivalents of bromine whereas an alkene adds only one equivalent. Other reactions are listed below. Alkynes are usually more reactive than alkenes. They show greater tendency to polymerize or oligomerize than alkenes do. The resulting polymers, called polyacetylenes (which do not contain alkyne units) are conjugated and can exhibit semiconducting properties.
Structure and bonding
In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes tend to be rod-like. Correspondingly, cyclic alkynes are rare. Benzyne is highly unstable. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C-C bond in alkanes (153 pm).
The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes 369 kJ/mol, the first pi bond contributes 268 kJ/mol and the second pi-bond of 202 kJ/mol bond strength. Bonding usually discussed in the context of molecular orbital theory, which recognizes the triple bond as arising from overlap of s and p orbitals. In the language of valence bond theory, the carbon atoms in an alkyne bond are sp hybridized: they each have two unhybridized p orbitals and two sp hybrid orbitals. Overlap of an sp orbital from each atom forms one sp-sp sigma bond. Each p orbital on one atom overlaps one on the other atom, forming two pi bonds, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom, for example to hydrogen atoms in the parent acetylene. The two sp orbitals project on opposite sides of the carbon atom.
Terminal and internal alkynes
Internal alkynes feature carbon substituents on each acetylenic carbon. Symmetrical examples include diphenylacetylene and 3-hexyne. A representative unsymmetrical internal alkyne is propiolic acid. Terminal alkynes have at least one hydrogen atom bonded to an sp hybridized carbon (those involved in the triple bond. An example would be methylacetylene (propyne using IUPAC nomenclature). Terminal alkynes and acetylene are mildly acidic. The acidic hydrogen on terminal alkynes can be replaced by a variety of groups resulting in halo-, silyl-, and alkoxoalkynes.
Synthesis
Commercially, the dominant alkyne is acetylene itself, which is used as a fuel and a precursor to other compounds, e.g., acrylates. Hundreds of millions of kilograms are produced annually by dehydrogenation of natural gas:
- 2 CH4 → HC≡CH + 3 H2
Propyne, also industrially useful, is also prepared by thermal cracking of hydrocarbons. Most other industrially useful alkyne derivatives are prepared from acetylene, e.g. via condensation with formaldehyde.[1]
Specialty alkynes are prepared by dehydrohalogenation of vicinal alkyl dihalides or vinyl halides.[2] Metal acetylides can be coupled with primary alkyl halides. Via the Fritsch-Buttenberg-Wiechell rearrangement, alkynes are prepared from vinyl bromides. Alkynes can be prepared from aldehydes using the Corey-Fuchs reaction and from aldehydes or ketones by the Seyferth–Gilbert homologation. In the alkyne zipper reaction, alkynes are generated from other alkynes by treatment with a strong base.
Reactions
Featuring a reactive functional group, alkynes participate in many organic reactions.
Alkynes characteristically undergo reactions that show that they are "doubly unsaturated," meaning that each alkyne unit is capable of adding two equivalents of H2, halogens or related HX reagents (X = halide, pseudohalide, etc.). Depending on catalysts and conditions, alkynes add one or two equivalents of hydrogen. Hydrogenation to the alkene is usually more desirable since alkanes are less useful:
- RC≡CR' + H2 → cis-RCH=CR'H
The largest scale application of this technology is the conversion of acetylene to ethylene in refineries. The steam cracking of alkanes affords a few percent acetylene, which is selectively hydrogenated in the presence of a palladium/silver catalyst. For more complex alkynes, the Lindlar catalyst is widely recommended to avoid formation of the alkane, for example in the conversion of phenylacetylene to styrene.[3]
Similarly, halogenation of alkynes gives the vinyl dihalides or alkyl tetrahalides:
- RC≡CR' + 2 Br2 → RCBr2CRBr2
The addition of nonpolar E-H bonds across C≡C is general for silanes, boranes, and related hydrides. The hydroboration of alkynes gives vinylic boranes which oxidize to the corresponding aldehyde or ketone. In the thiol-yne reaction the substrate is a thiol.
Hydrohalogenation gives the corresponding vinyl halides or alkyl dihalides, again depending on the number of equivalents of HX added. The addition of water to alkynes is a related reaction except the initial enol intermediate converts to the ketone or aldehyde. Illustrative is the hydration of phenylacetylene gives acetophenone,[4] and the (Ph3P)AuCH3-catalyzed hydration of 1,8-nonadiyne to 2,8-nonanedione:[5]
- PhC≡CH + H2O → PhCOCH3
- HC≡CC6H12C≡CH + 2H2O → CH3COC6H12COCH3
Cycloadditions and oxidation
Alkynes undergo diverse cycloaddition reactions. Most notable is the Diels–Alder reaction with 1,3-dienes to give 1,4-cyclohexadienes. This general reaction has been extensively developed and electrophilic alkynes are especially effective dienophiles. The "cycloadduct" derived from the addition of alkynes to 2-pyrone eliminates carbon dioxide to give the aromatic compound. Other specialized cycloadditions include multicomponent reactions such as alkyne trimerisation to give aromatic compounds and the [2+2+1]cycloaddition of an alkyne, alkene and carbon monoxide in the Pauson–Khand reaction. Non-carbon reagents also undergo cyclization, e.g. Azide alkyne Huisgen cycloaddition to give triazoles. Cycloaddition processes involving alkynes are often catalyzed by metals, e.g. enyne metathesis and alkyne metathesis, which allows the scrambling of carbyne (RC) centers:
- RC≡CR + R'C≡CR'
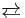 2 RC≡CR'
2 RC≡CR'
Oxidative cleavage of alkynes proceeds via cycloaddition to metal oxides. Most famously, potassium permanganate converts alkynes to a pair of carboxylic acids.
Reactions specific for terminal alkynes
In addition to undergoing the reactions characteristic of internal alkynes, terminal alkynes are reactive as weak acids, with pKa values (25) between that of ammonia (35) and ethanol (16). The acetylide conjugate base is stabilized as a result of the high s character of the sp orbital, in which the electron pair resides. Electrons in an s orbital benefit from closer proximity to the positively charged atom nucleus, and are therefore lower in energy. Treatment of terminal alkynes with a strong base gives the corresponding metal acetylides:
The reactions of alkynes with certain metal cations, e.g. Ag+ also gives acetylides. Thus, few drops of diamminesilver(I) hydroxide (Ag(NH3)2OH) reacts with terminal alkynes signaled by formation of a white precipitate of the silver acetylide. Acetylide derivatives are synthetically useful nucleophiles that participate in C-C bond forming reactions, as illustrated in the area called "Reppe Chemistry".
In the Favorskii reaction, terminal alkynes add to carbonyl compounds to give the hydroxyalkyne. Coupling of terminal alkynes to give di-alkynes is effected in the Cadiot-Chodkiewicz coupling, Glaser coupling, and the Eglinton coupling reactions. Terminal alkynes can also be coupled to aryl or vinyl halides as in the Sonogashira coupling.
Alkynes in nature and medicine
According to Ferdinand Bohlmann, the first naturally occurring acetylenic compound, dehydromatricaria ester, was isolated from an Artemisia species in 1826. In the nearly two centuries that have followed, well over a thousand naturally occurring acetylenes have been discovered and reported. Polyynes, a subset of this class of natural products, have been isolated from a wide variety of plant species, cultures of higher fungi, bacteria, marine sponges, and corals.[6] Some acids like tariric acid contains an alkyne group. Diynes and triynes, species with the linkage RC≡C-C≡CR' and RC≡C-C≡C-C≡CR' respectively, occur in certain plants (Ichthyothere, Chrysanthemum, Cicuta, Oenanthe and other members of the Asteraceae and Apiaceae families). Some examples are cicutoxin, oenanthotoxin, falcarinol and carotatoxin. These compounds are highly bioactive, e.g. as nematocides.[7] 1-Phenylhepta-1,3,5-triyne is illustrative of a naturally occurring triyne.
Alkynes occur in some pharmaceuticals, including the contraceptive norethynodrel. A carbon-carbon triple bond is also present in marketed drugs such as the antiretroviral Efavirenz and the antifungal Terbinafine. Molecules called ene-diynes feature a ring containing an alkene ("ene") between two alkyne groups ("diyne"). These compounds, e.g. calicheamicin, are some of the most aggressive antitumor drugs known, so much so that the ene-diyne subunit is sometimes referred to as a "warhead." Ene-diynes undergo rearrangement via the Bergman cyclization, generating highly reactive radical intermediates that attack DNA within the tumor.[8]
See also
References
- ^ Peter Pässler, Werner Hefner, Klaus Buckl, Helmut Meinass, Andreas Meiswinkel, Hans-Jürgen Wernicke, Günter Ebersberg, Richard Müller, Jürgen Bässler, Hartmut Behringer, Dieter Mayer (October 15, 2008). "Acetylene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_097.pub3.
- ^ Kenneth N. Campbell and Barbara K. Campbell (1963), "Phenylacetylene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0763; Coll. Vol. 4: 763
- ^ H. Lindlar and R. Dubuis (1973), "Palladium catalyst for partial reduction of acetylenes", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0880; Coll. Vol. 5: 880
- ^ Fukuda, Y.; Utimoto, K. (1991). "Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst". J. Org. Chem. 56 (11): 3729. doi:10.1021/jo00011a058.
- ^ Mizushima, E.; Cui, D.-M.; Nath, D. C. D.; Hayashi, T.; Tanaka, M. (2005), "Au(I)-Catalyzed hydratation of alkynes: 2,8-nonanedione", Org. Synth. 83: 55, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v83p0055
- ^ Annabelle L. K. Shi Shun; Rik R. Tykwinski (2006). "Synthesis of Naturally Occurring Polyynes". Angew. Chem. Int. Ed. 45 (7): 1034–1057. doi:10.1002/anie.200502071. PMID 16447152.
- ^ Lam, Jørgen (1988). Chemistry and biology of naturally-occurring acetylenes and related compounds (NOARC): proceedings of a Conference on the Chemistry and Biology of Naturally-Occurring Acetylenes and Related Compounds (NOARC). Amsterdam: Elsevier. ISBN 0-444-87115-2.
- ^ S. Walker; R. Landovitz; W.D. Ding; G.A. Ellestad; D. Kahne (1992). "Cleavage behavior of calicheamicin gamma 1 and calicheamicin T". Proc Natl Acad Sci U.S.A. 89 (10): 4608–12. doi:10.1073/pnas.89.10.4608. PMC 49132. PMID 1584797. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=49132.
|
|||||
|
|||||
|
||||||||
|
|||||||||||||||||