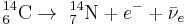Carbon-14
| Carbon-14 | |
|---|---|
| General | |
| Name, symbol | radiocarbon,14C |
| Neutrons | 8 |
| Protons | 6 |
| Nuclide data | |
| Natural abundance | 1 part per trillion |
| Half-life | 5,730 ± 40 years |
| Isotope mass | 14.003241 u |
| Spin | 0+ |
| Decay mode | Decay energy |
| Beta | 0.156476[1] MeV |
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949), to date archaeological, geological, and hydrogeological samples.
Carbon-14 was discovered on 27 February 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, although its existence had been suggested by Franz Kurie in 1934.[2]
There are three naturally occurring isotopes of carbon on Earth: 99% of the carbon is carbon-12, 1% is carbon-13, and carbon-14 occurs in trace amounts, i.e. making up as much as 1 part per trillion (0.0000000001%) of the carbon in the atmosphere. The half-life of carbon-14 is 5,730±40 years. Carbon-14 decays into nitrogen-14 through beta decay.[3] The primary natural source of carbon-14 on Earth is cosmic ray action upon nitrogen in the atmosphere, and it is therefore a cosmogenic nuclide. However, open-air nuclear testing between 1955-1980 contributed to this pool.
The different isotopes of carbon do not differ appreciably in their chemical properties. This is used in chemical and biological research, in a technique called carbon labeling: carbon-14 atoms can be used to replace nonradioactive carbon, in order to trace chemical and biochemical reactions involving carbon atoms from any given organic compound.
Contents |
Origin and radioactive decay
Carbon-14 is produced in the upper layers of the troposphere and the stratosphere by thermal neutrons absorbed by nitrogen atoms. When cosmic rays enter the atmosphere, they undergo various transformations, including the production of neutrons. The resulting neutrons (1n) participate in the following reaction:
- 1n + 14N → 14C + 1p
The highest rate of carbon-14 production takes place at altitudes of 9 to 15 km (30,000 to 50,000 ft) and at high geomagnetic latitudes, but the carbon-14 readily mixes and becomes evenly distributed throughout the atmosphere and reacts with oxygen to form radioactive carbon dioxide. Carbon dioxide also dissolves in water and thus permeates the oceans.
Carbon-14 then goes through radioactive beta decay.
By emitting an electron and an electron antineutrino, carbon-14 (half-life of 5730 years) decays into the stable (non-radioactive) isotope nitrogen-14.
The inventory of carbon-14 in Earth's biosphere is about 300 megacuries (11 EBq), of which most is in the oceans.[4]
As of 2008, the rate of carbon-14 production was not known - while the reaction can be modelled or the current concentrations and the global carbon budget can be used to backtrack, attempts to measure production had not agreed with these models. Production rates vary because of changes to the cosmic ray flux incident, such as supernovae, and due to variations in the Earth's magnetic field. The latter can create significant variations in carbon-14 production rates, although the changes of the carbon cycle can make these effects difficult to tease out.[5]
Other carbon-14 sources
Carbon-14 can also be produced by other neutron reactions, including in particular 13C(n,gamma)14C and 17O(n,alpha)14C with thermal neutrons, and 15N(n,d)14C and 16O(n,3He)14C with fast neutrons.[6]
Radiocarbon dating
Radiocarbon dating is a radiometric dating method that uses (14C) to determine the age of carbonaceous materials up to about 60,000 years old. The technique was developed by Willard Libby and his colleagues in 1949[7] during his tenure as a professor at the University of Chicago. Libby estimated that the radioactivity of exchangeable carbon-14 would be about 14 disintegrations per minute (dpm) per gram of pure carbon, and this is still used as the activity of the modern radiocarbon standard.[8][9] In 1960, Libby was awarded the Nobel Prize in chemistry for this work.
One of the frequent uses of the technique is to date organic remains from archaeological sites. Plants fix atmospheric carbon during photosynthesis, so the level of 14C in plants and animals when they die approximately equals the level of 14C in the atmosphere at that time. However, it decreases thereafter from radioactive decay, allowing the date of death or fixation to be estimated. The initial 14C level for the calculation can either be estimated, or else directly compared with known year-by-year data from tree-ring data (dendrochronology) up to 10,000 years ago (using overlapping data from live and dead trees in a given area), or else from cave deposits (speleothems), back to about 45,000 years before the present. A calculation or (more accurately) a direct comparison of carbon-14 levels in a sample, with tree ring or cave-deposit carbon-14 levels of a known age, then gives the wood or animal sample age-since-formation.
Formation during nuclear tests
The above-ground nuclear tests that occurred in several countries between 1955 and 1980 (see nuclear test list) dramatically increased the amount of carbon-14 in the atmosphere and subsequently in the biosphere; after the tests ended the atmospheric concentration of the isotope began to decrease.
One side effect of the change in atmospheric carbon-14 is that this has enabled some options for determining the birth year of an individual, in particular, the amount of carbon-14 in tooth enamel,[13][14] or the carbon-14 concentration in the lens of the eye.[15]
Occurrence
In fossil fuels
Most man-made chemicals are made of fossil fuels, such as petroleum or coal, in which the carbon-14 should have long since decayed. However, such deposits often contain trace amounts of carbon-14 (varying significantly, but ranging from 1% the ratio found in living organisms to amounts comparable to an apparent age of 40,000 years for oils with the highest levels of carbon-14).[16] This may indicate possible contamination by small amounts of bacteria, underground sources of radiation causing the 14N(n,p) 14C reaction, direct uranium decay (although reported measured ratios of 14C/U in uranium-bearing ores[17] would imply roughly 1 uranium atom for every two carbon atoms in order to cause the 14C/12C ratio, measured to be on the order of 10−15), or other unknown secondary sources of carbon-14 production. Presence of carbon-14 in the isotopic signature of a sample of carbonaceous material possibly indicates its contamination by biogenic sources or the decay of radioactive material in surrounding geologic strata. In connection with building the Borexino solar neutrino observatory, petroleum feedstock (for synthesizing the primary scintillant) was obtained with low 14C content. In the Borexino Counting Test Facility, a 14C/12C ratio of 1.94x10−18 was determined;[18] reactions responsible for varied levels of 14C in different petroleum reservoirs, and the lower 14C levels in methane, have been discussed by Bonvicini et al.[19]
In the human body
Since essentially all sources of human food are derived from plants, the carbon that comprises our bodies contains carbon-14 at the same concentration as the atmosphere. The rate of disintegrations of potassium-40 and carbon-14 in the normal adult body is comparable (a few thousand disintegrated nuclei per second).[20] The beta-decays from external (environmental) radiocarbon contribute approximately 0.01 mSv/year (1 mrem/year) to each person's dose of ionizing radiation.[21] This is small compared to the doses from potassium-40 (0.39 mSv/year) and radon (variable).
Carbon-14 can be used as a radioactive tracer in medicine. In the initial variant of the urea breath test, a diagnostic test for Helicobacter pylori, urea labeled with approximately 37 kBq (1.0 µCi) carbon-14 is fed to a patient (i.e. 37,000 decays per second). In the event of a H. pylori infection, the bacterial urease enzyme breaks down the urea into ammonia and radioactively-labeled carbon dioxide, which can be detected by low-level counting of the patient's breath.[22] The 14-C urea breath test has been largely replaced by the 13-C urea breath test which has no radiation issues.
See also
References
- ^ A.H Waptstra, G. Audi, and C. Thibault. "AME atomic mass evaluation 2003". http://www.nndc.bnl.gov/masses/mass.mas03. Retrieved 2007-06-03.
- ^ Kamen, Martin D. (1963). "Early History of Carbon-14: Discovery of this supremely important tracer was expected in the physical sense but not in the chemical sense". Science 140 (3567): 584–590. Bibcode 1963Sci...140..584K. doi:10.1126/science.140.3567.584. PMID 17737092.
- ^ "What is carbon dating?". National Ocean Sciences Accelerator Mass Spectrometry Facility. http://www.nosams.whoi.edu/about/carbon_dating.html. Retrieved 2007-06-11.
- ^ "Human Health Fact Sheet - Carbon 14". Argonne National Laboratory, EVS. August 2005. http://www.ead.anl.gov/pub/doc/carbon14.pdf.
- ^ Ramsey, C. Bronk (2008). "Radiocarbon Dating: Revolutions in Understanding". Archaeometry 50 (2): 249–275. doi:10.1111/j.1475-4754.2008.00394.x.
- ^ W. Davis Jr. "Carbon-14 production in nuclear reactors". ORNL/NUREG/TM-12. U.S. Nuclear Regulatory Commission. 1977
- ^ Arnold, J. R. and Libby, W. F. (1949). "Age Determinations by Radiocarbon Content: Checks with Samples of Known Age,". Science 110 (2869): 678–680. Bibcode 1949Sci...110..678A. doi:10.1126/science.110.2869.678. PMID 15407879.
- ^ "Carbon 14:age calculation". C14dating.com. http://www.c14dating.com/agecalc.html. Retrieved 2007-06-11.
- ^ "Class notes for Isotope Hydrology EESC W 4886: Radiocarbon 14C". Martin Stute's homepage at Columbia. http://www.ldeo.columbia.edu/~martins/isohydro/c_14.html. Retrieved 2007-06-11.
- ^ "Atmospheric δ14C record from Wellington". Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center (Oak Ridge National Laboratory). 1994. http://cdiac.esd.ornl.gov/trends/co2/welling.html. Retrieved 2007-06-11.
- ^ Levin, I., et al. (1994). "δ14C record from Vermunt". Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center. http://cdiac.esd.ornl.gov/trends/co2/cent-verm.html.
- ^ "Radiocarbon dating". University of Utrecht. http://www1.phys.uu.nl/ams/Radiocarbon.htm. Retrieved 2008-02-19.
- ^ "Radiation in Teeth Can Help Date, ID Bodies, Experts Say". National Geographic News. 2005-09-22. http://news.nationalgeographic.com/news/2005/09/0922_050922_nuke_body.html.
- ^ Spalding KL, Buchholz BA, Bergman LE, Druid H, Frisen J. (2005-09-15). "Forensics: age written in teeth by nuclear tests". Nature 437 (7057): 333–4. Bibcode 2005Natur.437..333S. doi:10.1038/437333a. PMID 16163340.
- ^ http://www.plosone.org/article/info:doi/10.1371/journal.pone.0001529 Niels Lynnerup, et al., Radiocarbon Dating of the Human Eye Lens Crystallines Reveal Proteins without Carbon Turnover throughout Life, Public Library of Science
- ^ D.C. Lowe, "Problems Associated with the Use of Coal as a Source of 14C Free Background Material," Radiocarbon, 1989, 31:117-120
- ^ Jull, A.J.T.; Barker, D., Donahue, D. J. (12 1985). "Carbon-14 Abundances in Uranium Ores and Possible Spontaneous Exotic Emission from U-Series Nuclides". Meteorics 20: 676. (abstract)
- ^ Alimonti, G.; et al. (1998). "Measurement of the 14C abundance in a low-background liquid scintillator". Physics Letters B 422 (1–4): 349–358. Bibcode 1998PhLB..422..349B. doi:10.1016/S0370-2693(97)01565-7.(abstract)
- ^ Bonvicini, G, Harris, N and Paolone, V, "The chemical history of 14C in deep oilfields", Aug 2003. (arXiv:hep-ex/0308025)
- ^ THE RADIOACTIVITY OF THE NORMAL ADULT BODY - http://www.rerowland.com/BodyActivity.htm
- ^ NCRP Report No. 93 (1987). Ionizing Radiation Exposure of the Population of the United States. National Council on Radiation Protection and Measurements. (excerpt)
- ^ "Society of Nuclear Medicine Procedure Guideline for C-14 Urea Breath Test" (PDF). 2001-06-23. http://interactive.snm.org/docs/pg_ch07_0403.pdf. Retrieved 2007-07-04.
Further reading
- Kamen, Martin D. (1985). Radiant Science, Dark Politics: A Memoir of the Nuclear Age. Berkeley: University of California Press. ISBN 0520049292.
External links
| Lighter: carbon-13 |
Carbon-14 is an isotope of carbon |
Heavier: carbon-15 |
| Decay product of: boron-14, nitrogen-18 |
Decay chain of Carbon-14 |
Decays to: nitrogen-14 |