Octanol
| Octanol | |
|---|---|
|
Octan-1-ol |
|
|
Other names
1-Octanol; Capryl alcohol; Octyl alcohol |
|
| Identifiers | |
| CAS number | 111-87-5 |
| PubChem | 957 |
| ChEMBL | CHEMBL26215 |
| Jmol-3D images | Image 1 |
|
|
| Properties | |
| Molecular formula | C8H18O |
| Molar mass | 130.23 g mol−1 |
| Density | 0.824 g/cm3 |
| Melting point |
-16 °C, 257 K, 3 °F |
| Boiling point |
195 °C, 468 K, 383 °F |
| Solubility in water | Insoluble |
| (verify) (what is: /?) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH3(CH2)7OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and 4-octanol.
Octanol occurs naturally in the form of esters in some essential oils. The primary use of octanol is in the manufacture of various esters (both synthetic and naturally occurring), such as octyl acetate, which are used in perfumery and flavors. Other uses include experimental medical applications utilizing octanol to control Essential Tremor and other types of involuntary neurological tremors.[1]
Preparation
Octanol is produced industrially by the oligomerization of ethylene using triethylaluminium followed by oxidation of the alkylaluminium products.[2] An idealized synthesis is shown:
- Al(C2H5)3 + 9 C2H4 → Al(C8H17)3
- Al(C8H17)3 + 3 O + 3 H2O → 3 HOC8H17 + Al(OH)3
The process generates a range of alcohols that are separated by distillation.
Water/octanol partitioning
Octanol and water are immiscible. The distribution of a compound between water and octanol is used to calculate the partition coefficient 'P' of that molecule (often expressed as its logarithm to the base 10, log P). Water/ octanol partitioning is a relatively good approximation of the partitioning between the cytosol and lipid membranes of living systems.[3]
Many dermal absorption models consider the stratum corneum/ water partition coefficient to be well approximated by a function of the water/ octanol partition coefficient of the form:[4]
Where a and b are constants,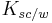 is the stratum corneum/ water partition coefficient, and
is the stratum corneum/ water partition coefficient, and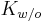 is the water/ octanol partition coefficient. The values of a and b vary between papers, but Cleek & Bunge[5] have reported the values a=0, b=0.74.
is the water/ octanol partition coefficient. The values of a and b vary between papers, but Cleek & Bunge[5] have reported the values a=0, b=0.74.
References
- ^ Bushara K. et al. (2004). "Pilot trial of 1-octanol in essential tremor". Neurology 62 (1): 122–124. PMID 14718713.
- ^ Jürgen Falbe, Helmut Bahrmann, Wolfgang Lipps, Dieter Mayer "Alcohols, Aliphatic" in Ullmann's Encyclopedia of Chemical Technology Wiley-VCH Verlag; Weinheim, 2002. doi:10.1002/14356007.a01_279
- ^ Schwarzenbach, Rene P.; Gschwend, Philip M.; Imboden, Dieter M. (2003). Environmental organic chemistry. John Wiley. ISBN 0471350532.
- ^ McCarley KD, Bunge AL (2001). "Pharmacokinetic Models of Dermal Absorption". Journal of Pharmaceutical Sciences 90 (11): 1699–1719. doi:10.1002/jps.1120. PMID 11745728.
- ^ Cleek RL, Bunge AL (1993). "A new method for estimating dermal absorption from chemical exposure. 1. General approach". Pharmaceutical Research 10 (4): 497–506. doi:10.1023/A:1018981515480. PMID 8483831.
|
|||||||||||||||||