Nuclear fusion
| Nuclear physics | ||||||||||||||
 |
||||||||||||||
| Radioactive decay Nuclear fission Nuclear fusion
|
||||||||||||||
In nuclear physics, nuclear chemistry, and astrophysics, nuclear fusion is the process in which two or more atomic nuclei join together to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy. Large scale thermonuclear fusion processes, involving many nuclei fusing at once, must occur in matter at very high densities and temperatures.
The fusion of two nuclei with lower masses than iron (which, along with nickel, has the largest binding energy per nucleon) generally releases energy while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission.
In the simplest case of hydrogen fusion, two protons have to be brought close enough for the weak nuclear force to convert either of the identical protons into a neutron forming the hydrogen isotope deuterium. In more complex cases of heavy ion fusion involving two or more nucleons, the reaction mechanism is different, but the same result occurs - one of combining smaller nuclei into larger nuclei.
Nuclear fusion occurs naturally in all active stars. See astrophysics. Synthetic fusion as a result of human actions has also been achieved, although this has not yet been completely controlled as a source of nuclear power. In the laboratory, successful nuclear physics experiments have been carried out that involve the fusion of many different varieties of nuclei, but the energy output has been negligible in these studies. In fact, the amount of energy put into the process has always exceeded the energy output.
Uncontrolled nuclear fusion has been carried out many times in nuclear weapons testing, which always results in a deliberate explosion. These explosions have always used the heavy isotopes of hydrogen, deuterium (H-2) and tritium (H-3), and never the much more common isotope of hydrogen (H-1), sometimes called "protium".
Building upon the nuclear transmutation experiments by Ernest Rutherford, carried out several years earlier, the fusion of the light nuclei (hydrogen isotopes) was first accomplished by Mark Oliphant in 1932. Then, the steps of the main cycle of nuclear fusion in stars were first worked out by Hans Bethe throughout the remainder of that decade.
Research into fusion for military purposes began in the early 1940s as part of the Manhattan Project, but this was not accomplished until 1951 (see the Greenhouse Item nuclear test), and nuclear fusion on a large scale in an explosion was first carried out on November 1, 1952, in the Ivy Mike hydrogen bomb test. Research into developing controlled thermonuclear fusion for civil purposes also began in the 1950s, and it continues to this day.
Contents |
Overview
Fusion reactions power the stars and produce virtually all elements in a process called nucleosynthesis. Although the fusion of lighter elements in stars releases energy, production of elements heavier than iron absorbs energy.
When the fusion reaction is a sustained uncontrolled chain, it can result in a thermonuclear explosion, such as that generated by a hydrogen bomb. Non-self sustaining reactions can still release considerable energy, as well as large numbers of neutrons.
Research into controlled fusion, with the aim of producing fusion power for the production of electricity, has been conducted for over 50 years. It has been accompanied by extreme scientific and technological difficulties, but has resulted in progress. At present, break-even (self-sustaining) controlled fusion reactions have not been demonstrated in the few tokamak-type reactors around the world.[2] Workable designs for a reactor that theoretically will deliver ten times more fusion energy than the amount needed to heat up plasma to required temperatures (see ITER) were originally scheduled to be operational in 2018, however this has been delayed and a new date has not been stated.
It takes considerable energy to force nuclei to fuse, even those of the lightest element, hydrogen. This is because all nuclei have a positive charge (due to their protons), and as like charges repel, nuclei strongly resist being put too close together. Accelerated to high speeds (that is, heated to thermonuclear temperatures), they can overcome this electromagnetic repulsion and get close enough for the attractive nuclear force to be sufficiently strong to achieve fusion. The fusion of lighter nuclei, which creates a heavier nucleus and a free neutron, generally releases more energy than it takes to force the nuclei together; this is an exothermic process that can produce self-sustaining reactions. The National Ignition Facility, which uses laser-driven inertial confinement fusion, is thought to be capable of break-even fusion. The first large-scale laser target experiments were performed in June 2009[3] and ignition experiments will begin in 2010.[3]
The energy released in most nuclear reactions is much larger than that in chemical reactions, because the binding energy that holds a nucleus together is far greater than the energy that holds electrons to a nucleus. For example, the ionization energy gained by adding an electron to a hydrogen nucleus is 13.6 eV—less than one-millionth of the 17 MeV released in the deuterium–tritium (D–T) reaction shown in the diagram to the right. Fusion reactions have an energy density many times greater than nuclear fission; the reactions produce far greater energies per unit of mass even though individual fission reactions are generally much more energetic than individual fusion ones, which are themselves millions of times more energetic than chemical reactions. Only direct conversion of mass into energy, such as that caused by the collision of matter and antimatter, is more energetic per unit of mass than nuclear fusion.
Requirements
A substantial energy barrier of electrostatic forces must be overcome before fusion can occur. At large distances two naked nuclei repel one another because of the repulsive electrostatic force between their positively charged protons. If two nuclei can be brought close enough together, however, the electrostatic repulsion can be overcome by the attractive nuclear force, which is stronger at close distances.
When a nucleon such as a proton or neutron is added to a nucleus, the nuclear force attracts it to other nucleons, but primarily to its immediate neighbours due to the short range of the force. The nucleons in the interior of a nucleus have more neighboring nucleons than those on the surface. Since smaller nuclei have a larger surface area-to-volume ratio, the binding energy per nucleon due to the nuclear force generally increases with the size of the nucleus but approaches a limiting value corresponding to that of a nucleus with a diameter of about four nucleons. It is important to keep in mind that the above picture is a toy model because nucleons are quantum objects, and so, for example, since two neutrons in a nucleus are identical to each other, distinguishing one from the other, such as which one is in the interior and which is on the surface, is in fact meaningless, and the inclusion of quantum mechanics is necessary for proper calculations.
The electrostatic force, on the other hand, is an inverse-square force, so a proton added to a nucleus will feel an electrostatic repulsion from all the other protons in the nucleus. The electrostatic energy per nucleon due to the electrostatic force thus increases without limit as nuclei get larger.

The net result of these opposing forces is that the binding energy per nucleon generally increases with increasing size, up to the elements iron and nickel, and then decreases for heavier nuclei. Eventually, the binding energy becomes negative and very heavy nuclei (all with more than 208 nucleons, corresponding to a diameter of about 6 nucleons) are not stable. The four most tightly bound nuclei, in decreasing order of binding energy, are 62Ni, 58Fe, 56Fe, and 60Ni.[4] Even though the nickel isotope ,62Ni, is more stable, the iron isotope 56Fe is an order of magnitude more common. This is due to a greater disintegration rate for 62Ni in the interior of stars driven by photon absorption.
A notable exception to this general trend is the helium-4 nucleus, whose binding energy is higher than that of lithium, the next heaviest element. The Pauli exclusion principle provides an explanation for this exceptional behavior—it says that because protons and neutrons are fermions, they cannot exist in exactly the same state. Each proton or neutron energy state in a nucleus can accommodate both a spin up particle and a spin down particle. Helium-4 has an anomalously large binding energy because its nucleus consists of two protons and two neutrons; so all four of its nucleons can be in the ground state. Any additional nucleons would have to go into higher energy states.
The situation is similar if two nuclei are brought together. As they approach each other, all the protons in one nucleus repel all the protons in the other. Not until the two nuclei actually come in contact can the strong nuclear force take over. Consequently, even when the final energy state is lower, there is a large energy barrier that must first be overcome. It is called the Coulomb barrier.
The Coulomb barrier is smallest for isotopes of hydrogen—they contain only a single positive charge in the nucleus. A bi-proton is not stable, so neutrons must also be involved, ideally in such a way that a helium nucleus, with its extremely tight binding, is one of the products.
Using deuterium-tritium fuel, the resulting energy barrier is about 0.01 MeV. In comparison, the energy needed to remove an electron from hydrogen is 13.6 eV, about 750 times less energy. The (intermediate) result of the fusion is an unstable 5He nucleus, which immediately ejects a neutron with 14.1 MeV. The recoil energy of the remaining 4He nucleus is 3.5 MeV, so the total energy liberated is 17.6 MeV. This is many times more than what was needed to overcome the energy barrier.
If the energy to initiate the reaction comes from accelerating one of the nuclei, the process is called beam-target fusion; if both nuclei are accelerated, it is beam-beam fusion. If the nuclei are part of a plasma near thermal equilibrium, the process is called thermonuclear fusion. Temperature is a measure of the average kinetic energy of particles, so by heating the nuclei they will gain energy and eventually have enough to overcome this 0.01 MeV. Converting the units between electronvolts and kelvin shows that the barrier would be overcome at a temperature in excess of 120 million kelvin.
There are two effects that lower the actual temperature needed. One is the fact that temperature is the average kinetic energy, implying that some nuclei at this temperature would actually have much higher energy than 0.01 MeV, while others would be much lower. It is the nuclei in the high-energy tail of the velocity distribution that account for most of the fusion reactions. The other effect is quantum tunneling. The nuclei do not actually have to have enough energy to overcome the Coulomb barrier completely. If they have nearly enough energy, they can tunnel through the remaining barrier. For this reason fuel at lower temperatures will still undergo fusion events, at a lower rate.

The reaction cross section σ is a measure of the probability of a fusion reaction as a function of the relative velocity of the two reactant nuclei. If the reactants have a distribution of velocities, e.g. a thermal distribution with thermonuclear fusion, then it is useful to perform an average over the distributions of the product of cross section and velocity. The reaction rate (fusions per volume per time) is <σv> times the product of the reactant number densities:
If a species of nuclei is reacting with itself, such as the DD reaction, then the product  must be replaced by
must be replaced by  .
.
 increases from virtually zero at room temperatures up to meaningful magnitudes at temperatures of 10–100 keV. At these temperatures, well above typical ionization energies (13.6 eV in the hydrogen case), the fusion reactants exist in a plasma state.
increases from virtually zero at room temperatures up to meaningful magnitudes at temperatures of 10–100 keV. At these temperatures, well above typical ionization energies (13.6 eV in the hydrogen case), the fusion reactants exist in a plasma state.
The significance of  as a function of temperature in a device with a particular energy confinement time is found by considering the Lawson criterion.
as a function of temperature in a device with a particular energy confinement time is found by considering the Lawson criterion.
Gravitational confinement
One force capable of confining the fuel well enough to satisfy the Lawson criterion is gravity. The mass needed, however, is so great that gravitational confinement is only found in stars (the least massive of which that are capable of fusion are red dwarfs). Even if the more reactive fuel deuterium were used, a mass greater than that of the planet Jupiter would be needed. In stars heavy enough, after the supply of hydrogen is exhausted in their cores, their cores (or a shell around the core) start fusing helium to carbon. In the most massive stars (at least 8-11 solar masses), the process is continued until some of their energy is produced by fusing lighter elements to iron. As iron has one of the highest binding energies, reactions producing heavier elements are generally endothermic. Therefore significant amounts of heavier elements are not formed during stable periods of massive star evolution, but are formed in supernova explosions and some lighter stars. Some of these heavier elements can in turn produce energy in nuclear fission.
Magnetic confinement
- See Magnetic confinement fusion for more information.
Electrically charged particles (such as fuel ions) will follow magnetic field lines (see Guiding center). The fusion fuel can therefore be trapped using a strong magnetic field. A variety of magnetic configurations exist, including the toroidal geometries of tokamaks and stellarators and open-ended mirror confinement systems.
Inertial confinement
- See Inertial fusion energy for more information.
A third confinement principle is to apply a rapid pulse of energy to a large part of the surface of a pellet of fusion fuel, causing it to simultaneously "implode" and heat to very high pressure and temperature. If the fuel is dense enough and hot enough, the fusion reaction rate will be high enough to burn a significant fraction of the fuel before it has dissipated. To achieve these extreme conditions, the initially cold fuel must be explosively compressed. Inertial confinement is used in the hydrogen bomb, where the driver is x-rays created by a fission bomb. Inertial confinement is also attempted in "controlled" nuclear fusion, where the driver is a laser, ion, or electron beam, or a Z-pinch. Another method is to use conventional high explosive material to compress a fuel to fusion conditions.[5][6] The UTIAS explosive-driven-implosion facility was used to produce stable, centered and focused hemispherical implosions[7] to generate neutrons from D-D reactions. The simplest and most direct method proved to be in a predetonated stoichiometric mixture of deuterium-oxygen. The other successful method was using a miniature Voitenko compressor,[8] where a plane diaphragm was driven by the implosion wave into a secondary small spherical cavity that contained pure deuterium gas at one atmosphere.[9]
Some confinement principles have been investigated, such as muon-catalyzed fusion, the Farnsworth–Hirsch fusor and Polywell (inertial electrostatic confinement), and bubble fusion.
Production methods
A variety of methods are known to effect nuclear fusion. Some are "cold" in the strict sense that no part of the material is hot (except for the reaction products), some are "cold" in the limited sense that the bulk of the material is at a relatively low temperature and pressure but the reactants are not, and some are "hot" fusion methods that create macroscopic regions of very high temperature and pressure.
Muon-catalyzed fusion
Muon-catalyzed fusion is a well-established and reproducible fusion process that occurs at ordinary temperatures. It was studied in detail by Steven Jones in the early 1980s. It has not been reported to produce net energy. Net energy production from this reaction cannot occur because of the energy required to create muons, their 2.2 µs half-life, and the chance that a muon will bind to the new alpha particle and thus stop catalyzing fusion.[10]
Generally cold, locally hot fusion
Accelerator-based light-ion fusion is a technique using particle accelerators to achieve particle kinetic energies sufficient to induce light-ion fusion reactions. Accelerating light ions is relatively easy, and can be done in an efficient manner—all it takes is a vacuum tube, a pair of electrodes, and a high-voltage transformer; fusion can be observed with as little as 10 kV between electrodes. The key problem with accelerator-based fusion (and with cold targets in general) is that fusion cross sections are many orders of magnitude lower than Coulomb interaction cross sections. Therefore the vast majority of ions end up expending their energy on bremsstrahlung and ionization of atoms of the target. Devices referred to as sealed-tube neutron generators are particularly relevant to this discussion. These small devices are miniature particle accelerators filled with deuterium and tritium gas in an arrangement that allows ions of these nuclei to be accelerated against hydride targets, also containing deuterium and tritium, where fusion takes place. Hundreds of neutron generators are produced annually for use in the petroleum industry where they are used in measurement equipment for locating and mapping oil reserves. Despite periodic reports in the popular press by scientists claiming to have invented "table-top" fusion machines, neutron generators have been around for half a century. The sizes of these devices vary but the smallest instruments are often packaged in sizes smaller than a loaf of bread. These devices do not produce a net power output.
Sonofusion or bubble fusion, a controversial variation on the sonoluminescence theme, suggests that acoustic shock waves, creating temporary bubbles (cavitation) that expand and collapse shortly after creation, can produce temperatures and pressures sufficient for nuclear fusion.[11]
The Farnsworth–Hirsch fusor is a tabletop device in which fusion occurs. This fusion comes from high effective temperatures produced by electrostatic acceleration of ions. The device can be built inexpensively, but it too is unable to produce a net power output.
The Polywell is a concept for a tabletop device in which fusion occurs. The device is a non-thermodynamic equilibrium machine that uses electrostatic confinement to accelerate ions into a center where they fuse together.
Antimatter-initialized fusion uses small amounts of antimatter to trigger a tiny fusion explosion. This has been studied primarily in the context of making nuclear pulse propulsion, and pure fusion bombs feasible. This is not near becoming a practical power source, due to the cost of manufacturing antimatter alone.
Pyroelectric fusion was reported in April 2005 by a team at UCLA. The scientists used a pyroelectric crystal heated from −34 to 7 °C (−29 to 45 °F), combined with a tungsten needle to produce an electric field of about 25 gigavolts per meter to ionize and accelerate deuterium nuclei into an erbium deuteride target. Though the energy of the deuterium ions generated by the crystal has not been directly measured, the authors used 100 keV (a temperature of about 109 K) as an estimate in their modeling.[12] At these energy levels, two deuterium nuclei can fuse together to produce a helium-3 nucleus, a 2.45 MeV neutron and bremsstrahlung. Although it makes a useful neutron generator, the apparatus is not intended for power generation since it requires far more energy than it produces.[13][14][15][16]
Hot fusion
In hot fusion, the fuel reaches tremendous temperature and pressure inside a fusion reactor or nuclear weapon (or star).
The methods in the second group are examples of non-equilibrium systems, in which very high temperatures and pressures are produced in a relatively small region adjacent to material of much lower temperature. In his doctoral thesis for MIT, Todd Rider did a theoretical study of all quasineutral, isotropic, non-equilibrium fusion systems. He demonstrated that all such systems will leak energy at a rapid rate due to bremsstrahlung produced when electrons in the plasma hit other electrons or ions at a cooler temperature and suddenly decelerate. The problem is not as pronounced in a hot plasma because the range of temperatures, and thus the magnitude of the deceleration, is much lower. Note that Rider's work does not apply to non-neutral and/or anisotropic non-equilibrium plasmas.
Important reactions
Astrophysical reaction chains
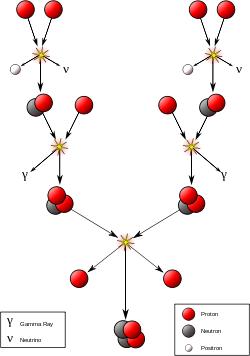

The most important fusion process in nature is the one that powers stars. The net result is the fusion of four protons into one alpha particle, with the release of two positrons, two neutrinos (which changes two of the protons into neutrons), and energy, but several individual reactions are involved, depending on the mass of the star. For stars the size of the sun or smaller, the proton-proton chain dominates. In heavier stars, the CNO cycle is more important. Both types of processes are responsible for the creation of new elements as part of stellar nucleosynthesis.
At the temperatures and densities in stellar cores the rates of fusion reactions are notoriously slow. For example, at solar core temperature (T ≈ 15 MK) and density (160 g/cm³), the energy release rate is only 276 μW/cm³—about a quarter of the volumetric rate at which a resting human body generates heat.[17] Thus, reproduction of stellar core conditions in a lab for nuclear fusion power production is completely impractical. Because nuclear reaction rates strongly depend on temperature (exp(−E/kT)), achieving reasonable energy production rates in terrestrial fusion reactors requires 10–100 times higher temperatures (compared to stellar interiors): T ≈ 0.1–1.0 GK.
Criteria and candidates for terrestrial reactions
In man-made fusion, the primary fuel is not constrained to be protons and higher temperatures can be used, so reactions with larger cross-sections are chosen. This implies a lower Lawson criterion, and therefore less startup effort. Another concern is the production of neutrons, which activate the reactor structure radiologically, but also have the advantages of allowing volumetric extraction of the fusion energy and tritium breeding. Reactions that release no neutrons are referred to as aneutronic.
To be a useful energy source, a fusion reaction must satisfy several criteria. It must:
- Be exothermic: This may be obvious, but it limits the reactants to the low Z (number of protons) side of the curve of binding energy. It also makes helium 4He the most common product because of its extraordinarily tight binding, although 3He and 3H also show up.
- Involve low Z nuclei: This is because the electrostatic repulsion must be overcome before the nuclei are close enough to fuse.
- Have two reactants: At anything less than stellar densities, three body collisions are too improbable. In inertial confinement, both stellar densities and temperatures are exceeded to compensate for the shortcomings of the third parameter of the Lawson criterion, ICF's very short confinement time.
- Have two or more products: This allows simultaneous conservation of energy and momentum without relying on the electromagnetic force.
- Conserve both protons and neutrons: The cross sections for the weak interaction are too small.
Few reactions meet these criteria. The following are those with the largest cross sections:
-
(1) 21D + 31T → 42He ( 3.5 MeV ) + n0 ( 14.1 MeV ) (2i) 21D + 21D → 31T ( 1.01 MeV ) + p+ ( 3.02 MeV ) 50% (2ii) → 32He ( 0.82 MeV ) + n0 ( 2.45 MeV ) 50% (3) 21D + 32He → 42He ( 3.6 MeV ) + p+ ( 14.7 MeV ) (4) 31T + 31T → 42He + 2 n0 + 11.3 MeV (5) 32He + 32He → 42He + 2 p+ + 12.9 MeV (6i) 32He + 31T → 42He + p+ + n0 + 12.1 MeV 51% (6ii) → 42He ( 4.8 MeV ) + 21D ( 9.5 MeV ) 43% (6iii) → 42He ( 0.5 MeV ) + n0 ( 1.9 MeV ) + p+ ( 11.9 MeV ) 6% (7i) 21D + 63Li → 2 42He + 22.4 MeV (7ii) → 32He + 42He + n0 + 2.56 MeV (7iii) → 73Li + p+ + 5.0 MeV (7iv) → 74Be + n0 + 3.4 MeV (8) p+ + 63Li → 42He ( 1.7 MeV ) + 32He ( 2.3 MeV ) (9) 32He + 63Li → 2 42He + p+ + 16.9 MeV (10) p+ + 115B → 3 42He + 8.7 MeV
| Nucleosynthesis |

|
| Related topics |
|
For reactions with two products, the energy is divided between them in inverse proportion to their masses, as shown. In most reactions with three products, the distribution of energy varies. For reactions that can result in more than one set of products, the branching ratios are given.
Some reaction candidates can be eliminated at once.[18] The D-6Li reaction has no advantage compared to p+-115B because it is roughly as difficult to burn but produces substantially more neutrons through 21D-21D side reactions. There is also a p+-73Li reaction, but the cross section is far too low, except possibly when Ti > 1 MeV, but at such high temperatures an endothermic, direct neutron-producing reaction also becomes very significant. Finally there is also a p+-94Be reaction, which is not only difficult to burn, but 94Be can be easily induced to split into two alpha particles and a neutron.
In addition to the fusion reactions, the following reactions with neutrons are important in order to "breed" tritium in "dry" fusion bombs and some proposed fusion reactors:
To evaluate the usefulness of these reactions, in addition to the reactants, the products, and the energy released, one needs to know something about the cross section. Any given fusion device has a maximum plasma pressure it can sustain, and an economical device would always operate near this maximum. Given this pressure, the largest fusion output is obtained when the temperature is chosen so that <σv>/T² is a maximum. This is also the temperature at which the value of the triple product nTτ required for ignition is a minimum, since that required value is inversely proportional to <σv>/T² (see Lawson criterion). (A plasma is "ignited" if the fusion reactions produce enough power to maintain the temperature without external heating.) This optimum temperature and the value of <σv>/T² at that temperature is given for a few of these reactions in the following table.
| fuel | T [keV] | <σv>/T² [m³/s/keV²] |
|---|---|---|
| 21D-31T | 13.6 | 1.24×10−24 |
| 21D-21D | 15 | 1.28×10−26 |
| 21D-32He | 58 | 2.24×10−26 |
| p+-63Li | 66 | 1.46×10−27 |
| p+-115B | 123 | 3.01×10−27 |
Note that many of the reactions form chains. For instance, a reactor fueled with 31T and 32He creates some 21D, which is then possible to use in the 21D-32He reaction if the energies are "right". An elegant idea is to combine the reactions (8) and (9). The 32He from reaction (8) can react with 63Li in reaction (9) before completely thermalizing. This produces an energetic proton, which in turn undergoes reaction (8) before thermalizing. Detailed analysis shows that this idea would not work well, but it is a good example of a case where the usual assumption of a Maxwellian plasma is not appropriate.
Neutronicity, confinement requirement, and power density

Any of the reactions above can in principle be the basis of fusion power production. In addition to the temperature and cross section discussed above, we must consider the total energy of the fusion products Efus, the energy of the charged fusion products Ech, and the atomic number Z of the non-hydrogenic reactant.
Specification of the 21D-21D reaction entails some difficulties, though. To begin with, one must average over the two branches (2) and (3). More difficult is to decide how to treat the 31T and 32He products. 31T burns so well in a deuterium plasma that it is almost impossible to extract from the plasma. The 21D-32He reaction is optimized at a much higher temperature, so the burnup at the optimum 21D-21D temperature may be low, so it seems reasonable to assume the 31T but not the 32He gets burned up and adds its energy to the net reaction. Thus we count the 21D-21D fusion energy as Efus = (4.03+17.6+3.27)/2 = 12.5 MeV and the energy in charged particles as Ech = (4.03+3.5+0.82)/2 = 4.2 MeV.
Another unique aspect of the 21D-21D reaction is that there is only one reactant, which must be taken into account when calculating the reaction rate.
With this choice, we tabulate parameters for four of the most important reactions
| fuel | Z | Efus [MeV] | Ech [MeV] | neutronicity |
|---|---|---|---|---|
| 21D-31T | 1 | 17.6 | 3.5 | 0.80 |
| 21D-21D | 1 | 12.5 | 4.2 | 0.66 |
| 21D-32He | 2 | 18.3 | 18.3 | ~0.05 |
| p+-115B | 5 | 8.7 | 8.7 | ~0.001 |
The last column is the neutronicity of the reaction, the fraction of the fusion energy released as neutrons. This is an important indicator of the magnitude of the problems associated with neutrons like radiation damage, biological shielding, remote handling, and safety. For the first two reactions it is calculated as (Efus-Ech)/Efus. For the last two reactions, where this calculation would give zero, the values quoted are rough estimates based on side reactions that produce neutrons in a plasma in thermal equilibrium.
Of course, the reactants should also be mixed in the optimal proportions. This is the case when each reactant ion plus its associated electrons accounts for half the pressure. Assuming that the total pressure is fixed, this means that density of the non-hydrogenic ion is smaller than that of the hydrogenic ion by a factor 2/(Z+1). Therefore the rate for these reactions is reduced by the same factor, on top of any differences in the values of <σv>/T². On the other hand, because the 21D-21D reaction has only one reactant, the rate is twice as high as if the fuel were divided between two hydrogenic species.
Thus there is a "penalty" of (2/(Z+1)) for non-hydrogenic fuels arising from the fact that they require more electrons, which take up pressure without participating in the fusion reaction. (It is usually a good assumption that the electron temperature will be nearly equal to the ion temperature. Some authors, however discuss the possibility that the electrons could be maintained substantially colder than the ions. In such a case, known as a "hot ion mode", the "penalty" would not apply.) There is at the same time a "bonus" of a factor 2 for 21D-21D because each ion can react with any of the other ions, not just a fraction of them.
We can now compare these reactions in the following table.
| fuel | <σv>/T² | penalty/bonus | reactivity | Lawson criterion | power density (W/m3/kPa2) | relation of power density |
|---|---|---|---|---|---|---|
| 21D-31T | 1.24×10−24 | 1 | 1 | 1 | 34 | 1 |
| 21D-21D | 1.28×10−26 | 2 | 48 | 30 | 0.5 | 68 |
| 21D-32He | 2.24×10−26 | 2/3 | 83 | 16 | 0.43 | 80 |
| p+-63Li | 1.46×10−27 | 1/2 | 1700 | 0.005 | 6800 | |
| p+-115B | 3.01×10−27 | 1/3 | 1240 | 500 | 0.014 | 2500 |
The maximum value of <σv>/T² is taken from a previous table. The "penalty/bonus" factor is that related to a non-hydrogenic reactant or a single-species reaction. The values in the column "reactivity" are found by dividing 1.24 × 10−24 by the product of the second and third columns. It indicates the factor by which the other reactions occur more slowly than the 21D-31T reaction under comparable conditions. The column "Lawson criterion" weights these results with Ech and gives an indication of how much more difficult it is to achieve ignition with these reactions, relative to the difficulty for the 21D-31T reaction. The last column is labeled "power density" and weights the practical reactivity with Efus. It indicates how much lower the fusion power density of the other reactions is compared to the 21D-31T reaction and can be considered a measure of the economic potential.
Bremsstrahlung losses in quasineutral, isotropic plasmas
The ions undergoing fusion in many systems will essentially never occur alone but will be mixed with electrons that in aggregate neutralize the ions' bulk electrical charge and form a plasma. The electrons will generally have a temperature comparable to or greater than that of the ions, so they will collide with the ions and emit x-ray radiation of 10-30 keV energy (Bremsstrahlung). The Sun and stars are opaque to x-rays, but essentially any terrestrial fusion reactor will be optically thin for x-rays of this energy range. X-rays are difficult to reflect but they are effectively absorbed (and converted into heat) in less than mm thickness of stainless steel (which is part of a reactor's shield). The ratio of fusion power produced to x-ray radiation lost to walls is an important figure of merit. This ratio is generally maximized at a much higher temperature than that which maximizes the power density (see the previous subsection). The following table shows the rough optimum temperature and the power ratio at that temperature for several reactions.[19]
| fuel | Ti (keV) | Pfusion/PBremsstrahlung |
|---|---|---|
| 21D-31T | 50 | 140 |
| 21D-21D | 500 | 2.9 |
| 21D-32He | 100 | 5.3 |
| 32He-32He | 1000 | 0.72 |
| p+-63Li | 800 | 0.21 |
| p+-115B | 300 | 0.57 |
The actual ratios of fusion to Bremsstrahlung power will likely be significantly lower for several reasons. For one, the calculation assumes that the energy of the fusion products is transmitted completely to the fuel ions, which then lose energy to the electrons by collisions, which in turn lose energy by Bremsstrahlung. However, because the fusion products move much faster than the fuel ions, they will give up a significant fraction of their energy directly to the electrons. Secondly, the plasma is assumed to be composed purely of fuel ions. In practice, there will be a significant proportion of impurity ions, which will then lower the ratio. In particular, the fusion products themselves must remain in the plasma until they have given up their energy, and will remain some time after that in any proposed confinement scheme. Finally, all channels of energy loss other than Bremsstrahlung have been neglected. The last two factors are related. On theoretical and experimental grounds, particle and energy confinement seem to be closely related. In a confinement scheme that does a good job of retaining energy, fusion products will build up. If the fusion products are efficiently ejected, then energy confinement will be poor, too.
The temperatures maximizing the fusion power compared to the Bremsstrahlung are in every case higher than the temperature that maximizes the power density and minimizes the required value of the fusion triple product. This will not change the optimum operating point for 21D-31T very much because the Bremsstrahlung fraction is low, but it will push the other fuels into regimes where the power density relative to 21D-31T is even lower and the required confinement even more difficult to achieve. For 21D-21D and 21D-32He, Bremsstrahlung losses will be a serious, possibly prohibitive problem. For 32He-32He, p+-63Li and p+-115B the Bremsstrahlung losses appear to make a fusion reactor using these fuels with a quasineutral, isotropic plasma impossible. Some ways out of this dilemma are considered—and rejected—in Fundamental limitations on plasma fusion systems not in thermodynamic equilibrium by Todd Rider.[20] This limitation does not apply to non-neutral and anisotropic plasmas; however, these have their own challenges to contend with.
See also
- Aneutronic fusion
- Focus fusion
- Fusion power
- Helium fusion
- Helium-3
- ITER
- National Ignition Facility
- Neutron generator
- Neutron source
- Nuclear fission
- Nuclear physics
- Nuclear reactor
- Nucleosynthesis
- Periodic table
- Polywell
- Pulsed power
- Solar surface fusion
- Stellar surface fusion
- Teller–Ulam design
- Timeline of nuclear fusion
References
- ↑ J.K. Shultis, R.E. Faw (2002). Fundamentals of nuclear science and engineering. CRC Press. p. 151. ISBN 0824708342. http://books.google.com/books?id=SO4Lmw8XoEMC&pg=PA151.
- ↑ "Progress in Fusion". ITER. http://www.iter.org/sci/Pages/BeyondITER.aspx. Retrieved 2010-02-15.
- ↑ "The National Ignition Facility: Ushering in a New Age for Science". National Ignition Facility. https://lasers.llnl.gov/programs/nif/. Retrieved 2009-09-13.
- ↑ The Most Tightly Bound Nuclei
- ↑ F. Winterberg"Conjectured Metastable Super-Explosives formed under High Pressure for Thermonuclear Ignition"
- ↑ Zhang, Fan (Medicine Hat, CA)Murray, Stephen Burke (Medicine Hat, CA)Higgins, Andrew (Montreal, CA)(2005)"Super compressed detonation method and device to effect such detonation"
- ↑ I.I. Glass and J.C. Poinssot"IMPLOSION DRIVEN SHOCK TUBE"
- ↑ D.Sagie and I.I. Glass(1982)"Explosive-driven hemispherical implosions for generating fusion plasmas"
- ↑ T. Saito, A. K. Kudian and I. I. Glass"Temperature Measurements Of An Implosion Focus"
- ↑ S.E. Jones (1986). "Muon-Catalysed Fusion Revisited". Nature 321: 127–133. doi:10.1038/321127a0.
- ↑ Access: Desktop fusion is back on the table: Nature News
- ↑ Supplementary methods for “Observation of nuclear fusion driven by a pyroelectric crystal”
- ↑ UCLA Crystal Fusion
- ↑ Physics News Update 729
- ↑ Coming in out of the cold: nuclear fusion, for real | csmonitor.com
- ↑ Nuclear fusion on the desktop ... really! - Science – MSNBC.com
- ↑ FusEdWeb | Fusion Education
- ↑ http://theses.mit.edu/Dienst/UI/2.0/Page/0018.mit.theses/1995-130/30?npages=306
- ↑ http://theses.mit.edu/Dienst/UI/2.0/Page/0018.mit.theses/1995-130/26?npages=306
- ↑ http://fusion.ps.uci.edu/artan/Posters/aps_poster_2.pdf Portable Document Format (PDF)
Further reading
- "What is Nuclear Fusion?". NuclearFiles.org. http://www.nuclearfiles.org/menu/key-issues/nuclear-weapons/basics/what-is-fusion.htm.
- S. Atzeni, J. Meyer-ter-Vehn (2004). "Nuclear fusion reactions". The Physics of Inertial Fusion. University of Oxford Press. ISBN 978-0-19-856264-1. http://www.oup.co.uk/pdf/0-19-856264-0.pdf.
- G. Brumfiel (22 May 2006). "Chaos could keep fusion under control". Nature. doi:10.1038/news060522-2.
- R.W. Bussard (9 November 2006). "Should Google Go Nuclear? Clean, Cheap, Nuclear Power". Google TechTalks. http://video.google.com/videoplay?docid=1996321846673788606&q=engedu.
- A. Wenisch, R. Kromp, D. Reinberger (November 2007). "Science of Fiction: Is there a Future for Nuclear?". Austrian Institute of Ecology. http://www.ecology.at/ecology/files/pr577_1.pdf.
- W.J. Nuttall (September 2008). "Fusion as an Energy Source: Challenges and Opportunities". Institute of Physics Report. Institute of Physics. http://www.iop.org/activity/policy/Publications/file_31695.pdf.
External links
- NuclearFiles.org – A repository of documents related to nuclear power.
- Organizations
- ITER (International Thermonuclear Experimental Reactor) website
- CCFE (Culham Centre for Fusion Energy) website
- JET (Joint European Torus) website
|
|||||
|
|||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||

