Tendon
| Tendon | |
|---|---|
 |
|
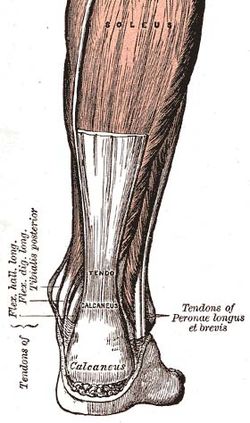
| One such tendon in the Human Body, the Achilles tendon. | |
| Latin | tendo |
| Dorlands/Elsevier | Tendon |
A tendon (or sinew) is a tough band of fibrous connective tissue that usually connects muscle to bone[1] and is capable of withstanding tension. Tendons are similar to ligaments and fasciae as they are all made of collagen except that ligaments join one bone to another bone, and fascia connect muscles to other muscles. Tendons and muscles work together and can only exert a pulling force.
Contents |
Structure
Normal healthy tendons are mostly composed of parallel arrays of collagen fibres closely packed together. The dry mass of normal tendons, which makes up about 30% of the total mass in water, is composed of about 86% collagen, 2% elastin, 1–5% proteoglycans, and 0.2% inorganic components such as copper, manganese, and calcium.[2][3] The collagen portion is made up of 97-98% type I collagen, with small amounts of other types of collagen. These include type II collagen in the cartilaginous zones, type III collagen in the reticulin fibres of the vascular walls, type IX collagen, type IV collagen in the basement membranes of the capillaries, type V collagen in the vascular walls, and type X collagen in the mineralized fibrocartilage near the interface with the bone.[2][4] Collagen fibres coalesce into macroaggregates. After secretion from the cell, the terminal peptides are cleaved by procollagen N- and C-proteinases, and the tropocollagen molecules spontaneously assemble into insoluble fibrils. A collagen molecule is about 300 nm long and 1-2 nm wide, and the diameter of the fibrils that are formed can range from 50-500 nm. In tendons, the fibrils then assemble further to form fascicles, which are about 10 mm in length with a diameter of 50-300 μm, and finally into a tendon fibre with a diameter of 100-500 μm.[5] Groups of fascicles are bounded by the epitendon and peritendon to form the tendon organ.
The collagen in tendons are held together with proteoglycan components, including decorin and, in compressed regions of tendon, aggrecan, which are capable of binding to the collagen fibrils at specific locations.[6] The proteoglycans are interwoven with the collagen fibrils and that their glycosaminoglycan (GAG) side chains have multiple interactions with the surface of the fibrils, showing that the proteoglycans are important structurally in the interconnection of the fibrils.[7] The major glycosaminoglycan (GAG) components of the tendon are dermatan sulfate and chondroitin sulfate, which associate with collagen and are involved in the fibril assembly process during tendon development. Dermatan sulfate is thought to be responsible for forming associations between fibrils, while chondroitin sulfate is thought to be more involved with occupying volume between the fibrils to keep them separated and help withstand deformation.[8] The dermatan sulfate side chains of decorin aggregate in solution, and this behavior can assist with the assembly of the collagen fibrils. When decorin molecules are bound to a collagen fibril, their dermatan sulfate chains may extend and associate with other dermatan sulfate chains on decorin that is bound to separate fibrils, therefore creating interfibrillar bridges and eventually causing parallel alignment of the fibrils.[9]
The tenocytes produce the collagen molecules which aggregate end-to-end and side-to-side to produce collagen fibrils. Fibril bundles are organized to form fibres with the elongated tenocytes closely packed between them. There is a three-dimensional network of cell processes associated with collagen in the tendon. The cells communicate with each other through gap junctions, and this signalling gives them the ability to detect and respond to mechanical loading.[10]
Blood vessels may be visualized within the endotendon running parallel to collagen fibres, with occasional branching transverse anastomoses.
The internal tendon bulk is thought to contain no nerve fibres, but the epi- and peritendon contain nerve endings, while Golgi tendon organs are present at the junction between tendon and muscle.
Tendon length varies in all major groups and from person to person. Tendon length is practically the discerning factor where muscle size and potential muscle size is concerned. For example, should all other relevant biological factors be equal, a man with a shorter tendons and a longer biceps muscle will have greater potential for muscle mass than a man with a longer tendon and a shorter muscle. Successful bodybuilders will generally have shorter tendons. Conversely, in sports requiring athletes to excel in actions such as running or jumping, it is beneficial to have longer than average Achilles tendon and a shorter calf muscle.[11]
Tendon length is determined by genetic predisposition, and has not been shown to either increase or decrease in response to environment, unlike muscles which can be shortened by trauma, use imbalances and a lack of recovery and stretching.[12]
Function
Tendons have been traditionally considered to simply be a mechanism by which muscles connect to bone, functioning simply to transmit forces. However, over the past two decades, much research focused on the elastic properties of tendons and their ability to function as springs. This allows tendons to passively modulate forces during locomotion, providing additional stability with no active work. It also allows tendons to store and recover energy at high efficiency. For example, during a human stride, the Achilles tendon stretches as the ankle joint dorsiflexes. During the last portion of the stride, as the foot plantar-flexes (pointing the toes down), the stored elastic energy is released. Furthermore, because the tendon stretches, the muscle is able to function with less or even no change in length, allowing the muscle to generate greater force.
The mechanical properties of the tendon are dependent on the collagen fiber diameter and orientation. The collagen fibrils are parallel to each other and closely packed, but show a wave-like appearance due to planar undulations, or crimps, on a scale of several micrometers.[13] In tendons, the collagen I fibres have some flexibility due to the absence of hydroxyproline and proline residues at specific locations in the amino acid sequence, which allows the formation of other conformations such as bends or internal loops in the triple helix and results in the development of crimps.[14] The crimps in the collagen fibrils allow the tendons to have some flexibility as well as a low compressive stiffness. In addition, because the tendon is a multi-stranded structure made up of many partially independent fibrils and fascicles, it does not behave as a single rod, and this property also contributes to its flexibility.[15]
The proteoglycan components of tendons also are important to the mechanical properties. While the collagen fibrils allow tendons to resist tensile stress, the proteoglycans allow them to resist compressive stress. The elongation and the strain of the collagen fibrils alone have been shown to be much lower than the total elongation and strain of the entire tendon under the same amount of stress, demonstrating that the proteoglycan-rich matrix must also undergo deformation, and stiffening of the matrix occurs at high strain rates.[16] These molecules are very hydrophilic, meaning that they can absorb a large amount of water and therefore have a high swelling ratio. Since they are noncovalently bound to the fibrils, they may reversibly associate and disassociate so that the bridges between fibrils can be broken and reformed. This process may be involved in allowing the fibril to elongate and decrease in diameter under tension.[17]
Mechanics
When stretched tendons have a soft tissue mechanical behavior.
Pathology
Tendons are subject to many types of injuries. There are various forms of tendinopathies or tendon injuries due to overuse. These types of injuries generally result in inflammation and degeneration or weakening of the tendons, which may eventually lead to tendon rupture.[18] Tendinopathies can be caused by a number of factors relating to the tendon extracellular matrix, and their classification has been difficult because their symptoms and histopathology often are similar. The first category of tendinopathy is paratenonitis, which refers to inflammation of the paratenon, or paratendinous sheet located between the tendon and its sheath. Tendinosis refers to non-inflammatory injury to the tendon at the cellular level. The degradation is caused by damage to collagen, cells, and the vascular components of the tendon, and is known to lead to rupture.[19] Observations of tendons that have undergone spontaneous rupture have shown the presence of collagen fibrils that are not in the correct parallel orientation or are not uniform in length or diameter, along with rounded tenocytes, other cell abnormalities, and the ingrowth of blood vessels.[18] Other forms of tendinosis that have not led to rupture have also shown the degeneration, disorientation, and thinning of the collagen fibrils, along with an increase in the amount of glycosaminoglycans between the fibrils.[20] The third is paratenonitis with tendinosis, in which combinations of paratenon inflammation and tendon degeneration are both present. The last is tendinitis which refers to degeneration with inflammation of the tendon as well as vascular disruption.[2]
Tendinopathies may be caused by several intrinsic factors including age, body weight, and nutrition. The extrinsic factors are often related to sports and include excessive forces or loading, poor training techniques, and environmental conditions.[21]
Healing
The tendons in the foot are highly complex and intricate. If any tendons break it is a long, painful healing process, not to mention the intricacy of the repairing (if fully severed) process. Most people that do not receive medical attention within the first 48 hours of the injury will suffer from severe swelling, pain, and an on-fire feeling where the injury occurred. They are very painful when they are inflamed or not in use.
It was believed previously that tendons could not undergo matrix turnover and that tenocytes were not capable of repair. However, it has been shown more recently that throughout the lifetime of a person, tenocytes in the tendon actively synthesize ECM components as well as enzymes such as matrix metalloproteinases (MMPs) can degrade the matrix.[21] Tendons are capable of healing and recovering from injuries in a process that is controlled by the tenocytes and their surrounding extracellular matrix. However, the healed tendons never regain the same mechanical properties as before the injury.
The three main stages of tendon healing are inflammation, repair or proliferation, and remodeling, which can be further divided into consolidation and maturation. These stages can overlap with each other. In the first stage, inflammatory cells such as neutrophils are recruited to the injury site, along with erythrocytes. Monocytes and macrophages are recruited within the first 24 hours, and phagocytosis of necrotic materials at the injury site occurs. After the release of vasoactive and chemotactic factors, angiogenesis and the proliferation of tenocytes are initiated. Tenocytes then move into the site and start to synthesize collagen III.[18][20] The inflammation stage usually lasts for a few days, and the repair or proliferation stage then begins. In this stage, which lasts for about six weeks, the tenocytes are involved in the synthesis of large amounts of collagen and proteoglycans at the site of injury, and the levels of GAG and water are high.[22] After about six weeks, the remodeling stage begins. The first part of the remodeling stage is consolidation, which lasts from about six to ten weeks after the injury. During this time, the synthesis of collagen and GAGs is decreased, and the cellularity is also decreased as the tissue becomes more fibrous as a result of increased production of collagen I and the fibrils become aligned in the direction of mechanical stress.[20] The final maturation stage occurs after ten weeks, and during this time there is an increase in crosslinking of the collagen fibrils, which causes the tissue to become stiffer. Gradually, over a time period of about one year, the tissue will turn from fibrous to scar-like.[22]
Matrix metalloproteinases or MMPs have a very important role in the degradation and remodeling of the ECM during the healing process after a tendon injury. Certain MMPs including MMP-1, MMP-2, MMP-8, MMP-13, and MMP-14 have collagenase activity, meaning that unlike many other enzymes, they are capable of degrading collagen I fibrils. The degradation of the collagen fibrils by MMP-1 along with the presence of denatured collagen are factors that are believed to cause weakening of the tendon ECM and an increase in the potential for another rupture to occur.[23] In response to repeated mechanical loading or injury, cytokines may be released by tenocytes and can induce the release of MMPs, causing degradation of the ECM and leading to recurring injury and chronic tendinopathies.[20]
A variety of other molecules are involved in tendon repair and regeneration. There are five growth factors that have been shown to be significantly upregulated and active during tendon healing: insulin-like growth factor 1 (IGF-I), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and transforming growth factor beta (TGF-β).[22] These growth factors all have different roles during the healing process. IGF-1 increases collagen and proteoglycan production during the first stage of inflammation, and PDGF is also present during the early stages after injury and promotes the synthesis of other growth factors along with the synthesis of DNA and the proliferation of tendon cells.[22] The three isoforms of TGF-β (TGF-β1, TGF-β2, TGF-β3) are known to play a role in wound healing and scar formation.[24] VEGF is well known to promote angiogenesis and to induce endothelial cell proliferation and migration, and VEGF mRNA has been shown to be expressed at the site of tendon injuries along with collagen I mRNA.[25] Bone morphogenetic proteins (BMPs) are a subgroup of TGF-β superfamily that can induce bone and cartilage formation as well as tissue differentiation, and BMP-12 specifically has been shown to influence formation and differentiation of tendon tissue and to promote fibrogenesis.
Effects of activity on healing
In animal models, extensive studies have been conducted to investigate the effects of mechanical strain in the form of activity level on tendon injury and healing. While stretching can disrupt healing during the initial inflammatory phase, it has been shown that controlled movement of the tendons after about one week following an acute injury can help to promote the synthesis of collagen by the tenocytes, leading to increased tensile strength and diameter of the healed tendons and fewer adhesions than tendons that are immobilized. In chronic tendon injuries, mechanical loading has also been shown to stimulate fibroblast proliferation and collagen synthesis along with collagen realignment, all of which promote repair and remodeling.[22] To further support the theory that movement and activity assist in tendon healing, it has been shown that immobilization of the tendons after injury often has a negative effect on healing. In rabbits, collagen fascicles that are immobilized have shown decreased tensile strength, and immobilization also results in lower amounts of water, proteoglycans, and collagen crosslinks in the tendons.[18]
Several mechanotransduction mechanisms have been proposed as reasons for the response of tenocytes to mechanical force that enable them to alter their gene expression, protein synthesis, and cell phenotype and eventually cause changes in tendon structure. A major factor is mechanical deformation of the extracellular matrix, which can affect the actin cytoskeleton and therefore affect cell shape, motility, and function. Mechanical forces can be transmitted by focal adhesion sites, integrins, and cell-cell junctions. Changes in the actin cytoskeleton can activate integrins, which mediate “outside-in” and “inside-out” signaling between the cell and the matrix. G-proteins, which induce intracellular signaling cascades, may also be important, and ion channels are activated by stretching to allow ions such as calcium, sodium, or potassium to enter the cell.[22]
Ossified tendons
In some organisms, notably birds[26] and ornithischian dinosaurs[27], portions of the tendon can become ossified. In this process, osteocytes inflitrate the tendon and lay down bone as they would in sesamoid bone such as the patella. In birds, tendon ossification primarily occurs in the hindlimb, while in ornithischian dinosaurs, ossified axial muscle tendons form a latticework along the neural and haemal spines on the tail, presumably for support.
Uses of sinew
Sinew was widely used throughout pre-industrial eras as a tough, durable fiber. Some specific uses include using sinew as thread for sewing, attaching feathers to arrows (see fletch), lashing tool blades to shafts, etc. It is also recommended in survival guides as a material from which strong cordage can be made for items like traps or living structures. Tendon must be treated in specific ways to function usefully for these purposes. Inuit and other circumpolar people utilized sinew as the only cordage for all domestic purposes due to the lack of other suitable fiber sources in their ecological habitats.
The elastic properties of particular sinews were also used in composite recurved bows favoured by the steppe nomads of Eurasia. The first stone throwing artillery also used the elastic properties of sinew.
Sinew makes for an excellent cordage material for three reasons: It is incredibly strong, it contains natural glues, and it shrinks as it dries, doing away with the need for knots.
Tendon (particularly beef tendon) is used as a food in some Asian cuisines (often served at Yum Cha or Dim Sum restaurants). One popular dish is Suan Bao Niu Jin, where the tendon is marinated in garlic. It is also sometimes found in the Vietnamese noodle dish phở.
See also
- Aponeurosis
- Chordae tendineae
- Cartilage
- Tendon sheath
References
- ↑ eMedicine/Stedman Medical Dictionary Lookup!
- ↑ 2.0 2.1 2.2 Jozsa, L., and Kannus, P., Human Tendons: Anatomy, Physiology, and Pathology. Human Kinetics: Champaign, IL, 1997.
- ↑ Lin, T. W.; Cardenas, L.; Soslowsky, L. J., (2004). "Biomechanics of tendon injury and repair.". Journal of Biomechanics 37 (6): 865-877. doi:10.1016/j.jbiomech.2003.11.005. PMID 15111074.
- ↑ Fukuta, S.; Oyama, M.; Kavalkovich, K.; Fu, F. H.; Niyibizi, C., (1998). "Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon.". Matrix Biology 17 (1): 65-73. doi:10.1016/S0945-053X(98)90125-1. PMID 9628253.
- ↑ Fratzl, P. (2003). "Cellulose and collagen: from fibres to tissues.". Current Opinion in Colloid & Interface Science 8 (1): 32-39. doi:10.1016/S1359-0294(03)00011-6.
- ↑ Zhang, G. E., Y.; Chervoneva, I.; Robinson, P. S.; Beason, D. P.; Carine, E. T.; Soslowsky, L. J.; Iozzo, R. V.; Birk, D. E., (2006). "Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development.". Journal of Cellular Biochemistry 98 (6): 1436-1449. doi:10.1002/jcb.20776. PMID 16518859.
- ↑ Raspanti, M.; Congiu, T.; Guizzardi, S., (2002). "Structural Aspects of the Extracellular Matrix of the Tendon : An Atomic Force and Scanning Electron Microscopy Study.". Archives of Histology and Cytology 65 (1): 37-43. doi:10.1679/aohc.65.37. PMID 12002609.
- ↑ Scott, J. E. O., C. R.; Hughes, E. W., (1981). "Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation.". Biochemical Journal 195 (3): 573-581. PMID 6459082.
- ↑ Scott, J. E., (2003). "Elasticity in extracellular matrix 'shape modules' of tendon, cartilage, etc. A sliding proteoglycan-filament model.". Journal of Physiology 553 (2): 335-343. doi:10.1113/jphysiol.2003.050179. PMID 12923209.
- ↑ McNeilly, C. M.; Banes, A. J.; Benjamin, M.; Ralphs, J. R., (1996). "Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions.". Journal of Anatomy 189: 593-600. PMID 8982835.
- ↑ "Having a short Achilles tendon may be an athlete's Achilles heel". http://www.sportsinjurybulletin.com/archive/achilles-tendon.html. Retrieved 2007-10-26.
- ↑ Michael Young. "A Review on Postural Realignment and its Muscular and Neural Components". http://www.elitetrack.com/article_files/posture.pdf.
- ↑ Hulmes, D. J. S., (2002). "Building Collagen Molecules, Fibrils, and Suprafibrillar Structures.". Journal of Structural Biology 137 (1-2): 2-10. doi:10.1006/jsbi.2002.4450. PMID 12064927.
- ↑ Silver, F. H.; Freeman, J. W.; Seehra, G. P., (2003). "Collagen self-assembly and the development of tendon mechanical properties.". Journal of Biomechanics 36 (10): 1529-1553. doi:10.1016/S0021-9290(03)00135-0. PMID 14499302.
- ↑ Ker, R. F., (2002). "The implications of the adaptable fatigue quality of tendons for their construction, repair and function.". Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 133 (4): 987-1000. doi:10.1016/S1095-6433(02)00171-X. PMID 12485688.
- ↑ Puxkandl, R.; Zizak, I.; Paris, O.; Keckes, J.; Tesch, W.; Bernstorff, S.; Purslow, P.; Fratzl, P., (2002). "Viscoelastic properties of collagen: synchrotron radiation investigations and structural model.". Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 357 (1418): 191-197. doi:10.1098/rstb.2001.1033. PMID 11911776.
- ↑ Cribb, A. M.; Scott, J. E. In Tendon response to tensile-stress - an ultrastructural investigation of collagen - proteoglycan interactions in stressed tendon, 1995; Cambridge Univ Press: 1995; pp 423-428.
- ↑ 18.0 18.1 18.2 18.3 Sharma, P. M., N., (2006). "Biology of tendon injury: healing, modeling and remodeling.". Journal of Musculoskeletal and Neuronal Interactions 6 (2): 181-190. PMID 16849830.
- ↑ Astrom, M.; Rausing, A., (1995). "Chronic Achilles Tendinopathy - A survey of Surgical and Histopathologic findings.". Clinical Orthopaedics and Related Research 316 (316): 151-164. PMID 7634699.
- ↑ 20.0 20.1 20.2 20.3 Sharma, P.; Maffulli, N., (2005). "Tendon injury and tendinopathy: Healing and repair.". Journal of Bone and Joint Surgery-American Volume 87A (1): 187-202. doi:10.2106/JBJS.D.01850. PMID 15634833.
- ↑ 21.0 21.1 Riley, G., (2004). "The pathogenesis of tendinopathy. A molecular perspective.". Rheumatology 43 (2): 131-142. doi:10.1093/rheumatology/keg448. PMID 12867575.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 Wang, J. H. C., (2006). "Mechanobiology of tendon.". Journal of Biomechanics 39 (9): 1563-1582. doi:10.1016/j.jbiomech.2005.05.011. PMID 16000201.
- ↑ Riley, G. P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B. L.; Bank, R. A., (2002). "Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology.". Matrix Biology 21 (2): 185-195. doi:10.1016/S0945-053X(01)00196-2. PMID 11852234.
- ↑ Moulin, V.; Tam, B. Y. Y.; Castilloux, G.; Auger, F. A.; O'Connor-McCourt, M. D.; Philip, A.; Germain, L., (2001). "Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity.". Journal of Cellular Physiology 188 (2): 211-222. doi:10.1002/jcp.1110. PMID 11424088.
- ↑ Boyer, M. I. W., J. T.; Lou, J.; Manske, P. R.; Gelberman, R. H.; Cai, S. R., (2001). "Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model.". Journal of Orthopaedic Research 19 (5): 869-872. doi:10.1016/S0736-0266(01)00017-1. PMID 11562135.
- ↑ Berge, James C. Vanden; Storer, Robert W. (1995). "Intratendinous ossification in birds: A review". Journal of Morphology 226: 47. doi:10.1002/jmor.1052260105.
- ↑ Organ, Chris L. (2006). "Biomechanics of ossified tendons in ornithopod dinosaurs". Paleobiology 32: 652. doi:10.1666/05039.1.
|
|||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
