Tannin


Tannins are astringent, bitter plant polyphenols that either bind and precipitate or shrink proteins and various other organic compounds including amino acids and alkaloids. The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit or red wine.[1] Likewise, the destruction or modification of tannins with time plays an important role in the ripening of fruit and the aging of wine.
The term tannin (from tanna, an Old High German word for oak or fir tree, as in Tannenbaum) refers to the use of wood tannins from oak in tanning animal hides into leather; hence the words "tan" and "tanning" for the treatment of leather. However, the term "tannin" by extension is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with proteins and other macromolecules. The compounds are widely distributed in many species of plants, where they play a role in protection from predation, and perhaps also in growth regulation.
Tannins have molecular weights ranging from 500 to over 3,000.[2] Tannins are incompatible with alkalis, gelatin, heavy metals, iron, lime water, metallic salts, strong oxidizing agents and zinc sulfate, since they form complexes and precipitate in aqueous solution.
Tannins are usually divided into hydrolyzable tannins and condensed tannins (proanthocyanidins):
| Base Unit: | 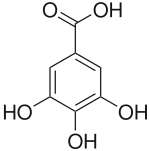 |
 |
|---|---|---|
| Class/Polymer: | Hydrolyzable tannins | Condensed tannins |
Contents |
Occurrence
Tannins are distributed in species throughout the plant kingdom. They are commonly found in both gymnosperms as well as angiosperms. Botanically, tannins are mainly physically located in the vacuoles or surface wax of plants. These storage sites keep tannins active against plant predators, but also keep some tannins from affecting plant metabolism while the plant tissue is alive; it is only after cell breakdown and death that the tannins are active in metabolic effects.
Tannins are found in leaf, bud, seed, root, and stem tissues. An example of the location of the tannins in stem tissue is that they are often found in the growth areas of trees, such as the secondary phloem and xylem and the layer between the cortex and epidermis. Tannins may help regulate the growth of these tissues.
There may be a loss in the bio-availability of still other tannins in plants due to birds, pests, and other pathogens.[3]
The leaching of tannins from the decaying leaves of vegetation adjoining a stream may produce what is known as a blackwater river.
Classes of Tannins
There are three major classes of tannins[4]: 1. Hydrolyzable tannins 2. Non-Hydrolyzable tannins or condensed tannins 3. Pseudotannins
Hydrolyzable Tannin
They are tannins on heating with hydrochloric or sulphuric acids yield gallic or ellagic acids.[4] At the center of a hydrolyzable tannin molecule, there is a carbohydrate (usually D-glucose but also cyclitols like quinic or shikimic acids). The hydroxyl groups of the carbohydrate are partially or totally esterified with phenolic groups such as gallic acid in gallotannins or ellagic acid in ellagitannins. Hydrolysable tannins are mixtures of polygalloyl glucoses and/or poly-galloyl quinic acid derivatives containing in between 3 up to 12 gallic acid residues per molecule[5].
Hydrolyzable tannins are hydrolyzed by weak acids or weak bases to produce carbohydrate and phenolic acids.
Examples of gallotannins are the gallic acid esters of glucose in tannic acid (C76H52O46), found in the leaves and bark of many plant species.
Non-Hydrolyzable or Condensed Tannin
They are tannins on heating with hydrochloric acid yield phlobaphenes like phloroglucinol.[4] Condensed tannins, also known as proanthocyanidins, are polymers of 2 to 50 (or more) flavonoid units that are joined by carbon-carbon bonds, which are not susceptible to being cleaved by hydrolysis. While hydrolyzable tannins and most condensed tannins are water soluble, some very large condensed tannins are insoluble.
Condensed tannins from Lithocarpus glaber leaves have been analysed through acid-catalyzed degradation in the presence of cysteamine and have a potent free radical scavenging activity[6].
Pseudo Tannins
Pseudo tannins are low molecular weight compounds associated with other compounds. They do not answer gold beater skin test unlike hydrolysable and condensed tannins.[4] (When gold beater skin or ox skin is dipped in HCl & treated with 1% FeSO4 solution, after washing with water it gives a blue / black colour). They are found in tea or coffee[7] or in : [8]
- Gallic acid : rhubarb
- Catechins : Acacia, catechu, cocoa, guarana
- Chlorogenic acid : Nux-vomica, coffee, mate
- Ipecacuanhic acid : Ipecacuanha
Nutrition
Tannins have traditionally been considered antinutritional but it is now known that their beneficial or antinutritional properties depend upon their chemical structure and dosage. The new technologies used to analyze molecular and chemical structures have shown that a division into condensed and hydrolyzable tannins is far too simplistic.[9] Recent studies have demonstrated that products containing chestnut tannins included at low dosages (0.15-0.2 %) in the diet can be beneficial.[10] Some studies suggest that chestnut tannins have been shown to have positive effects on silage quality in the round bale silages, in particular reducing ammonia and NPN (non protein nitrogen) in the lowest wilting level.[11] Improved fermentability of soya meal nitrogen in the rumen has also been reported by Mathieu F and Jouany JP (1993).[12] Studies by Gonzalez S. et al. (2002)[13] on in vitro ammonia release and dry matter degradation of soybean meal comparing three different types of tannins (quebracho, acacia and chestnut) demonstrated that chestnut tannins are more efficient in protecting soybean meal from in vitro degradation by rumen bacteria.
Condensed tannins inhibit herbivore digestion by binding to consumed plant proteins and making them more difficult for animals to digest, and by interfering with protein absorption and digestive enzymes (for more on that topic, see plant defense against herbivory).
Many tannin-consuming animals secrete a tannin-binding protein (mucin) in their saliva. Tannin-binding capacity of salivary mucin is directly related to its proline content. Advantages in using salivary proline-rich proteins (PRPs) to inactivate tannins are :
- PRPs inactivate tannins to a greater extent than do dietary proteins; this results in reduced fecal nitrogen losses,
- PRPs contain non specific nitrogen and nonessential amino acids; this makes them more convenient for an animal to exploit rather than using up valuable dietary protein.
Drinks with tannins
Coffee
Tannins were not found in any bean sample, and in contrast to previous reports, hydrolysable tannins sensu strictu were not detected in pulp. The presence of soluble condensed tannins in Coffea arabica pulp was confirmed at approximately 1%[14].
Tea


The tea plant (Camellia sinensis) is an example of a plant said to have a naturally high tannin content. When any type of tea leaf is steeped in hot water it brews a "tart" (astringent) flavor that is characteristic of tannins. This is due to the catechins and other flavonoids, which are categorized as tannins by doctors, biologists, and chemists.[15][16][17][18][19][20] Tea extracts have been reported to contain no tannic acid.[21]
Wine
Tannins are found in wine, particularly in red wine.
The tannins in wine are from two sources: firstly from the grape seeds, skins and stems, and secondly from the oak barrels (typically Quercus petraea or "French oak") in which wine is aged.
A simplistic summary often heard is that the tannins from the skin/seeds/stems are "bad" (that is, harsh and difficult to deal with), whereas the tannins from the wood are "good" (arguably desirable in the ultimate product, and easier to deal with).
Tannins in grape skins and seeds (the latter being especially harsh) tend to be more noticeable in red wines, which are macerated (soaked with skins and seeds) and sometimes fermented while in contact with the skins and seeds to extract the color from the skins. The stems of the grape bunches also contain tannins, and will also contribute tannins if the bunches are not stemmed before winemaking.
Tannins from grapes are condensed tannins, which are polymers of proanthocyanidin monomers.
Hydrolyzable tannins are extracted from the barrel wood. Hydrolyzable tannins are more easily oxidized than condensed tannins.
Indeed, as wine ages (that is to say, in the bottle), generally speaking the "good" tannins from the wood will slowly go away over the years.
Thus, if a wine is too "tannic," it is often said to be a good idea to leave it in the cellar for another (for example) five or ten years. Of course, this will typically only "work" with the "good," hydrolyzable tannins which came from the oak barrels used during the winemaking process; any "bad" tannins will (typically) be just as prominent in five or ten years: there is no simple catch-all formula for wine aging.
Modern winemakers take great care to minimize undesirable tannins from seeds by crushing grapes gently when extracting their juice, to avoid crushing the seeds. Pressing the grapes further results in press wine which contains more tannin and might be kept separately. Stemming is also widely practiced. Wines can also take on tannins if matured in oak or wood casks with a high tannin content. Tannins play an important role in preventing oxidation in aging wine and appear to polymerize and make up a major portion of the sediment in wine.
In recent years - since about the 1980s - there has been a tendency to drink red wine far younger than in the past. Indeed before about 1980 red wine was rarely if ever drunk with less than ten years of age in the bottle; it would have been considered not ready. In contrast red wine today, both Burgundy and Bordeaux and other reds, are often drunk with as little as two years in the bottle. This dramatically affects the entire question of what is acceptable, indeed good, in terms of the tannin question.
A further complication is that tannins (the wood tannins) are in some contexts seen as a good thing, a mark of prestige, since - very simply - the wood tannins prove the wine was made in oak barrels, rather than made cheaply in steel vats.
A study in wine production and consumption has shown that tannins, in the form of proanthocyanidins, have a beneficial effect on vascular health. The study showed that tannins suppressed production of the peptide responsible for hardening arteries. To support their findings, the study also points out that wines from the regions of southwest France and Sardinia are particularly rich in proanthocyanidins, and that these regions also produce populations with longer life spans.[22]
Reactions of tannins and anthocyanins with the phenolic compound anthocyanidins creates another class of tannins known as pigmented tannins which influences the color of red wine[23]. Commercial preparations of tannins, known as enological tannins, made from oak wood, grape seed and skin, plant gall, chestnut, quebracho, gambier[24] and myrobalan fruits[25], can be added at different stages of the wine production to improve color durability.
Effects of tannins on the drinkability and aging potential of wine
Tannins are a natural preservative in wine. Un-aged wines with high tannin content can be less palatable than wines with a lower level of tannins. Tannins can be described as leaving a dry and puckered feeling with a "furriness" in the mouth that can be compared to a stewed tea, which is also very tannic. This effect is particularly profound when drinking tannic wines without the benefit of food.
Many wine lovers see natural tannins (found particularly in varietals such as Cabernet Sauvignon and often accentuated by heavy oak barrel aging) as a sign of potential longevity and ageability. Tannins impart a mouth-puckering astringency when the wine is young but "resolve" (through a chemical process called polymerization) into delicious and complex elements of "bottle bouquet" when the wine is cellared under appropriate temperature conditions, preferably in the range of a constant 55 to 60 °F (13 to 16 °C).[26] Such wines mellow and improve with age with the tannic "backbone" helping the wine survive for as long as 40 years or more. In many regions (such as in Bordeaux), tannic grapes such as Cabernet Sauvignon are blended with lower-tannin grapes such as Merlot or Cabernet Franc, diluting the tannic characteristics. White wines and wines that are vinified to be drunk young (for examples, see nouveau wines) typically have lower tannin levels.
Beer
In addition to the alpha acids extracted from hops to provide bitterness in beer, condensed tannins are also present. These originate both from the malt and hops. Especially in Germany, trained brewmasters consider the presence of tannins as a flaw. However, in some styles, the presence of this astringency is acceptable or even desired, as, for example, in a Flanders red ale. In lager type beers the tannins can form a precipitate with specific haze forming proteins in the beer resulting in turbidity at low temperature. This chill haze can be prevented by removing part of the tannins or part of the haze forming proteins. Tannins are removed using PVPP, haze forming proteins by using silica or tannic acid[27].
Citrus, fruit juices
Although citrus fruits do not themselves contain tannins, orange-colored juices often contain food dyes with tannins. Apple juice, grape juices and berry juices are all high in tannins. Sometimes tannins are even added to juices and ciders to create a more astringent feel to the taste.
Food Items with Tannins
Fruits
Pomegranates



Pomegranates contain a diverse array of tannins, particularly hydrolysable tannins. The most abundant of pomegranate tannins are called punicalagins. Punicalagins have a molecular weight of 1038 and are the largest molecule found intact in rat plasma after oral ingestion[28] and were found to show no toxic effects in rats who were given a 6% diet of punicalagins for 37 days.[29] Punicalagins are also found to be the major component responsible for pomegranate juice's antioxidant and health benefits.[30]
Several dietary supplements and nutritional ingredients are available that contain extracts of whole pomegranate and/or are standardized to punicalagins, the marker compound of pomegranate. Extracts of pomegranate are also Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration. It has been recommended to look for pomegranate ingredients that mimic the polyphenol ratio of the fruit, as potent synergistic effects have been observed in 'natural spectrum' extracts, especially pomegranate concentrate normalized to punicalagins.[31]
Persimmons
Some persimmons are highly astringent and therefore inedible when they are not extremely ripe (specifically the Korean, American, and Hachiya or Japanese). This is due to the high level of tannins, and if eaten by humans (and many other animals), the mouth will become completely dry, yet the saliva glands will continue to secrete saliva which cannot affect the tannin-laced food.
Berries
Most berries, such as cranberries,[32] strawberries and blueberries,[33] contain both hydrolyzable and condensed tannins.
Nuts
Nuts that can be consumed raw such as Hazelnuts, Walnuts and Pecans, contain high amounts of tannins. Almonds feature a lower content. Tannin concentration in the crude extract of these nuts did not directly translate to the same relationships for the condensed fraction [34] Peanuts without shells have a very low tannin content. Acorns contain such high concentrations of tannins that they need to be processed before they can be consumed safely.
The areca nut also contains tannin which contributes to its antibacterial properties.
Smoked foods
Tannins from the wood of mesquite, cherry, oak and other woods used in smoking are present on the surface of smoked fish and meat (although smoke from cherry wood can be toxic to humans.)
Herbs/spices
Cloves, tarragon, cumin, thyme, vanilla, and cinnamon all contain tannins.[35]
Legumes
Most legumes contain tannins. Red-colored beans contain the most tannins, and white-colored beans have the least. Chickpeas (also known as garbanzo beans) have a smaller amount of tannins.[36]
Chocolate
Chocolate liquor contains about 6% tannins.[37]
Toxicity
If ingested in excessive quantities, tannins inhibit the absorption of minerals such as iron which may, if prolonged, lead to anemia.[38] This is because tannins are metal ion chelators, and tannin-chelated metal ions are not bioavailable. Tannins have been shown to precipitate proteins,[2] which inhibits in some ruminant animals the absorption of nutrients from high-tannin grains such as sorghum. Tannins only reduce the bioavailability of plant sources of iron, also known as non-heme. Animal sources, or heme iron absorption will not be affected by tannins. Tannic acid does not affect absorption of other trace minerals such as zinc, copper, and manganese in rats.[39]
Tannins are phenolic compounds and interfere with iron absorption through a complex formation with iron when it is in the gastrointestinal lumen which decreases the bioavailability of iron. There is an important difference in the way in which the phenolic compounds interact with different hydroxylation patterns (gallic acid, catechin, chlorogenic acid) and the effect on iron absorption. The content of the iron-binding galloyl groups may be the major determinant of the inhibitory effect of phenolic compounds. However, condensed tannins do not interfere with iron absorption.[38]
In order to prevent these problems, it is advised to drink tea and coffee between meals, not during. Foods rich in vitamin C help neutralize tannin's effects on iron absorption. Adding lemon juice to tea will reduce the negative effect of tannins in iron absorption as well. Adding milk to coffee and tea has very little to no influence on the inhibitory effect of tannins.[40]
In sensitive individuals, a large intake of tannins may cause bowel irritation, kidney irritation, liver damage, irritation of the stomach and gastrointestinal pain. With the exception of tea, long-term and/or excessive use of herbs containing high concentrations of tannins is not recommended. A correlation has been made between esophogeal or nasal cancer in humans and regular consumption of certain herbs with high tannin concentrations.[41]
Many plants employ tannins to deter animals. It has not been determined whether tannin was produced for another purpose, e.g. as pesticide, or whether it evolved specifically for the purpose of inhibiting predation.[42] Animals that consume excessive amounts of these plants fall ill or die. Acorns are a well known problem in cattle breeding. The lethal dose is said to be around 6% of the animal's body weight. This is only an approximate figure since acorns from Red Oak were shown to contain on average two to four times the tannins than those from White Oak[43]. Some deer and moose were found to have perished due to ingesting acorns. Symptoms include ataxia and shortness of breath. Some animals, like squirrels and mule deer have developed the ability to consume high concentrations of tannins without ill effects. Humans would usually find the bitter taste of foods containing high amounts of tannins unpalatable. (Some humans were found to be unable to taste bitter foods.) Tannins are leeched from acorns before they are used for human consumption.
Uses
Tannins are an important ingredient in the process of tanning leather. Oak bark, mimosa and quebracho tree have traditionally been the primary source of tannery tannin, though inorganic tanning agents are also in use today and account for 90% of the world's leather production[44].
Tannins produce different colors with ferric chloride (either blue, blue black, or green to greenish black) according to the type of tannin. Iron gall ink is produced by treating a solution of tannins with iron(II) sulfate.
Tannin is a component in a type of industrial particleboard adhesive[45] developed jointly by the Tanzania Industrial Research and Development Organization and Forintek Labs Canada.
Tannins can be used for production of anti-corrosive primer, sold under brand name-Nox Primer for treatment of rusted steel surfaces prior to painting, rust converter to transform oxidized steel into a smooth sealed surface and rust inhibitor.
Medical uses and potential
Tannins may be employed medicinally in antidiarrheal, hemostatic, and antihemorrhoidal compounds
The anti-inflammatory effect of tannins help control all indications of gastritis, esophagitis, enteritis, and irritating bowel disorders. Diarrhea is also treated with an effective astringent medicine that does not stop the flow of the disturbing substance in the stomach; rather, it controls the irritation in the small intestine.
Tannins not only heal burns and stop bleeding, but they also stop infection while they continue to heal the wound internally. The ability of tannins to form a protective layer over the exposed tissue keeps the wound from being infected even more. Tannins are also beneficial when applied to the mucosal lining of the mouth.
Tannins can also be effective in protecting the kidneys. Tannins have been used for immediate relief of sore throats, diarrhea, dysentery, hemorrhaging, fatigue, skin ulcers and as a cicatrizant on gangrenous wounds. Tannins can cause regression of tumors that are already present in tissue, but if used excessively over time, they can cause tumors in healthy tissue. Tannins are used indirectly as molluscicides to interrupt the transmission cycle of schistosomiasis. They have also been reported to have anti-viral effects. When incubated with red grape juice and red wines with a high content of condensed tannins, the poliovirus, herpes simplex virus, and various enteric viruses are inactivated.[46]
Tannins are sometimes used to treat poisons from poison oak or from bee stings, causing instant relief.
Tannins have shown potential antiviral,[47][48][49] antibacterial[50][51] and antiparasitic effects.[52] In the past few years tannins have also been studied for their potential effects against cancer through different mechanisms.[53] [54] [55]
Tannins, including gallo and ellagic acid (epigallitannins), are inhibitors of HIV replication.
- 1,3,4-Tri-O-galloylquinic acid
- 3,5-di-O-galloyl-shikimic acid
- 3,4,5-tri-O-galloylshikimic acid
- punicalin
- punicalagin
inhibited HIV replication in infected H9 lymphocytes with little cytotoxicity. Two compounds, punicalin and punicacortein C, inhibited purified HIV reverse transcriptase.[56]
It is believed that tannins isolated from the stem bark of Myracrodruon urundeuva are of neuroprotective functions capable of reversing 6-hydroxydopamine induced toxicity. The plant has shown promising futures for therapeutic use, which may be of benefit to neuro disease patients [57]. Souza et al. discovered that the tannins isolated from the stem bark also has the antiinflammatory and antiulcer potency on rodents, showing a strong anioxidant property for possible therapeutic applications [58].
Retailers
- OmniChem NaturalSpecialities, a subsidiary of the Ajinomoto group is specializing in the tannin production[59]
References
Notes
- ↑ McGee, Harold (2004). On food and cooking: the science and lore of the kitchen. New York: Scribner. p. 714. ISBN 0-684-80001-2.
- ↑ 2.0 2.1 Bate-Smith and Swain, 1962, Flavonoid compounds. In : Comparative biochemistry. Florkin M. Mason H.S. Eds. Vol III. 75-809. Academic Press, New-York.
- ↑ Kadam, S. S.; Salunkhe, D. K.; Chavan, J. K. (1990). Dietary tannins: consequences and remedies. Boca Raton: CRC Press. p. 177. ISBN 0-8493-6811-1.
- ↑ 4.0 4.1 4.2 4.3 Notes on Tannins from PharmaXChange.info
- ↑ Pentagalloyl glucose on www.natural-specialities.com
- ↑ HPLC, NMR and MALDI-TOF MS analysis of condensed tannins from Lithocarpus glaber leaves with potent free radical scavenging activity. Liang Liang Zhang and Yi Ming Lin, 2008
- ↑ Tannins, Unkown author
- ↑ Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar
- ↑ Muller-Harvey I, McAllan AB (1992). "Tannins: Their biochemistry and nutritional properties". Adv. Plant Cell Biochem. And Biotechnol. 1: 151–217.
- ↑ Schiavone A, Guo K, Tassone S, et al. (March 2008). "Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks". Poult. Sci. 87 (3): 521–7. doi:10.3382/ps.2007-00113. PMID 18281579. http://ps.fass.org/cgi/pmidlookup?view=long&pmid=18281579.
- ↑ Tabacco E, Borreani G, Crovetto GM, Galassi G, Colombo D, Cavallarin L (1 December 2006). "Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage". J. Dairy Sci. 89 (12): 4736–46. doi:10.3168/jds.S0022-0302(06)72523-1. PMID 17106105. http://jds.fass.org/cgi/pmidlookup?view=long&pmid=17106105.
- ↑ Mathieu F and Jouany JP (1993). Effect of chestnut tannin on the fermentability of soyabean meal nitrogen in the rumen. Ann Zootech 42, 127
- ↑ González S, Pabón ML, Carulla J (2002). "Effects of tannins on in vitro ammonia release and dry matter degradation of soybean meal". Arch. Latinoam. Prod. Anim. 10 (2): 97–101.
- ↑ Tannins in wet-processed coffee beans and coffee pulp. M. N. Clifford and J. R. Ramirez-Martinez, Food Chemistry, Volume 40, Issue 2, 1991, Pages 191-200, doi:10.1016/0308-8146(91)90102-T
- ↑ Michiyo TSUJIMURA (1963), "On the Tannin in Tea Leaf", Proceedings of the Japan Academy 39 (6), http://www.journalarchive.jst.go.jp/jnlpdf.php?cdjournal=pjab1945&cdvol=39&noissue=6&startpage=376&lang=en&from=jnlabstract
- ↑ S. R. EVELYN, E. A. MAIHS AwD D. G. ROUX (1960), "Condensed Tannins THE OXIDATIVE CONDENSATION OF (+ )-CATECHIN", Biochem. J. 76: 27, http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1204594&blobtype=pdf
- ↑ Muramatsu K, Fukuyo M, Hara Y (1986), "Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats.", J Nutr Sci Vitaminol (Tokyo). 32
- ↑ Tej K. Bhat∗ , Bhupinder Singh & Om P. Sharma, "Microbial degradation of tannins – A current perspective", Biodegradation 9, http://www.springerlink.com/content/g5337700w0n1t7h0/fulltext.pdf?page=1
- ↑ Julia F. Morton (1972), "Further Associations of Plant Tannins and Human Cancer", Pharmaceutical Biology 12 (1)
- ↑ J. L. Mangan (1988), "NUTRITIONAL EFFECTS OF TANNINS IN ANIMAL FEEDS", Nutrition Research Reviews, http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=592272
- ↑ Wheeler, S.R. Tea and Tannins Science 204: 6-8 (1979) [1] as cited in
Yam TS, Hamilton-Miller JM, Shah S (August 1998). "The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2' synthesis, and beta-lactamase production in Staphylococcus aureus". J. Antimicrob. Chemother. 42 (2): 211–6. doi:10.1093/jac/42.2.211. PMID 9738838. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=9738838. - ↑ Corder R, Mullen W, Khan NQ, et al. (November 2006). "Oenology: red wine procyanidins and vascular health". Nature 444 (7119): 566. doi:10.1038/444566a. PMID 17136085. http://www.nature.com/nature/journal/v444/n7119/abs/444566a.html.
- ↑ Compositional investigation of pigmented tannin. Kennedy James A. and Hayasaka Yoji, A.C.S. symposium series, 2004, vol. 886, pp. 247-264
- ↑ Identification of the origin of commercial enological tannins by the analysis of monosaccharides and polyalcohols. Luz Sanz M., Martinez-Castro Isabel and Moreno-Arribas M. Victoria, Food chemistry, 2008, vol. 111, no3, pp. 778-783
- ↑ Preliminary Investigation for the Differentiation of Enological Tannins According to Botanical Origin: Determination of Gallic Acid and Its Derivatives. Marie-hélène Salagoity-Auguste, Christian Tricard, Frédéric Marsal and Pierre Sudraud, Am. J. Enol. Vitic. 37:4:301-303 (1986)
- ↑ Wine Lovers Page - Wine Lexicon: Tannic, tannis
- ↑ http://www.natural-specialities.com/natural-specialities/PDF/Applications/BR02%20overview%20fact%20sheet%20version%202.1.pdf
- ↑ Scalbert A, Morand C, Manach C, Rémésy C (August 2002). "Absorption and metabolism of polyphenols in the gut and impact on health". Biomed. Pharmacother. 56 (6): 276–82. doi:10.1016/S0753-3322(02)00205-6. PMID 12224598.
- ↑ Cerdá B, Cerón JJ, Tomás-Barberán FA, Espín JC (May 2003). "Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic". J. Agric. Food Chem. 51 (11): 3493–501. doi:10.1021/jf020842c. PMID 12744688.
- ↑ Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA (October 2000). "Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing". J. Agric. Food Chem. 48 (10): 4581–9. doi:10.1021/jf000404a. PMID 11052704.
- ↑ Seeram NP, Adams LS, Henning SM, et al. (June 2005). "In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice". J. Nutr. Biochem. 16 (6): 360–7. doi:10.1016/j.jnutbio.2005.01.006. PMID 15936648. http://linkinghub.elsevier.com/retrieve/pii/S0955-2863(05)00019-7.
- ↑ Vattem DA, Ghaedian R, Shetty K (2005). "Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry". Asia Pac J Clin Nutr 14 (2): 120–30. PMID 15927928. http://scholar.google.com/url?sa=U&q=http://www.nupro.net/science/enhnce%2520berry%2520phenoli.pdf.
- ↑ Puupponen-Pimiä R, Nohynek L, Meier C, et al. (April 2001). "Antimicrobial properties of phenolic compounds from berries". J. Appl. Microbiol. 90 (4): 494–507. doi:10.1046/j.1365-2672.2001.01271.x. PMID 11309059. http://www.blackwell-synergy.com/links/doi/10.1046/j.1365-2672.2001.01271.x/abs/.
- ↑ http://74.125.47.132/search?q=cache:K-qF4vdf8a8J:www.icomst.helsinki.fi/icomst2008/Paper%2520CD/General%2520speakers%2Bposters-3p%2520papers/Session2/2B/2B.3.Amarowicz.pdf+tannin+%22nuts%22&hl=en&ct=clnk&cd=10&gl=us
- ↑ Navia, Jeanette. “Could Tannins Explain Classic Migraine Triggers?” 1988
- ↑ Reed JD (1 May 1995). "Nutritional toxicology of tannins and related polyphenols in forage legumes". J. Anim. Sci. 73 (5): 1516–28. PMID 7665384. http://jas.fass.org/cgi/pmidlookup?view=long&pmid=7665384.
- ↑ Wolke, Robert L. (2005). What Einstein Told His Cook 2, The Sequel: Further Adventures in Kitchen Science (Hardcover). New York: W. W. Norton & Company. p. 433. ISBN 0-393-05869-7. [2]
- ↑ 38.0 38.1 Brune M, Rossander L, Hallberg L (August 1989). "Iron absorption and phenolic compounds: importance of different phenolic structures". Eur J Clin Nutr 43 (8): 547–57. PMID 2598894.
- ↑ Afsana K, Shiga K, Ishizuka S, Hara H (1 November 2003). "Ingestion of an indigestible saccharide, difructose anhydride III, partially prevents the tannic acid-induced suppression of iron absorption in rats". J. Nutr. 133 (11): 3553–60. PMID 14608073. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=14608073.
- ↑ Hurrell RF, Reddy M, Cook JD (April 1999). "Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages". Br. J. Nutr. 81 (4): 289–95. PMID 10999016. http://journals.cambridge.org/abstract_S0007114599000537.
- ↑ Elvin-Lewis, Memory P. F.; Lewis, Walter Hepworth (1977). Medical botany: plants affecting man's health. New York: Wiley. ISBN 0-471-53320-3.
- ↑ Katie E. Ferrell; Thorington, Richard W. (2006). Squirrels: the animal answer guide. Baltimore: Johns Hopkins University Press. p. 91. ISBN 0-8018-8402-0.
- ↑ Wood MD (2005). "Tannin and Lipid Content of Acorns in Scatterhoards and Larderhoards". Northeastern Naturalist 12 (4): 463–72. doi:10.1656/1092-6194(2005)012[0463:TALCOA]2.0.CO;2. "Figure 2: Mean tannin levels of red oak and white oak acorns retrieved after a period of storage.".
- ↑ Conservation of leather and related materials by M. Kite and R.Thomson, p. 23 (Elsevier 2005)
- ↑ Characterisation of tannin resin blends for particle board applications. E. T. N. Bisanda, W. O. Ogola and J. V. Tesha, Cement and Concrete Composites, Volume 25, Issue 6, August 2003, Pages 593-598
- ↑ Bajaj, Y. P. S. (1988). Medicinal and aromatic plants. Biotechnology in agriculture and forestry. 24. Berlin: Springer-Verlag. ISBN 0-387-56008-4.
- ↑ Cheng HY, Lin CC, Lin TC (September 2002). "Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn". Antiviral Res. 55 (3): 447–55. doi:10.1016/S0166-3542(02)00077-3. PMID 12206882. http://linkinghub.elsevier.com/retrieve/pii/S0166354202000773.
- ↑ Quideau S, Varadinova T, Karagiozova D, et al. (February 2004). "Main structural and stereochemical aspects of the antiherpetic activity of nonahydroxyterphenoyl-containing C-glycosidic ellagitannins". Chem. Biodivers. 1 (2): 247–58. doi:10.1002/cbdv.200490021. PMID 17191843.
- ↑ Lü L, Liu SW, Jiang SB, Wu SG (February 2004). "Tannin inhibits HIV-1 entry by targeting gp41". Acta Pharmacol. Sin. 25 (2): 213–8. PMID 14769212.
- ↑ Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K (October 2001). "Antibacterial action of several tannins against Staphylococcus aureus". J. Antimicrob. Chemother. 48 (4): 487–91. doi:10.1093/jac/48.4.487. PMID 11581226. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11581226.
- ↑ Funatogawa K, Hayashi S, Shimomura H, et al. (2004). "Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori". Microbiol. Immunol. 48 (4): 251–61. PMID 15107535. http://joi.jlc.jst.go.jp/JST.JSTAGE/mandi/48.251?from=PubMed.
- ↑ Kolodziej H, Kiderlen AF (September 2005). "Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells". Phytochemistry 66 (17): 2056–71. doi:10.1016/j.phytochem.2005.01.011. PMID 16153409. http://linkinghub.elsevier.com/retrieve/pii/S0031-9422(05)00012-9.
- ↑ Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW (March 1999). "p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells". Cancer Lett. 136 (2): 215–21. doi:10.1016/S0304-3835(98)00323-1. PMID 10355751. http://linkinghub.elsevier.com/retrieve/pii/S0304-3835(98)00323-1.
- ↑ Yang LL, Lee CY, Yen KY (August 2000). "Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells". Cancer Lett. 157 (1): 65–75. doi:10.1016/S0304-3835(00)00477-8. PMID 10893444. http://linkinghub.elsevier.com/retrieve/pii/S0304-3835(00)00477-8.
- ↑ Tanimura S, Kadomoto R, Tanaka T, Zhang YJ, Kouno I, Kohno M (May 2005). "Suppression of tumor cell invasiveness by hydrolyzable tannins (plant polyphenols) via the inhibition of matrix metalloproteinase-2/-9 activity". Biochem. Biophys. Res. Commun. 330 (4): 1306–13. doi:10.1016/j.bbrc.2005.03.116. PMID 15823585. http://linkinghub.elsevier.com/retrieve/pii/S0006-291X(05)00625-X.
- ↑ Nonaka G, Nishioka I, Nishizawa M, et al. (1990). "Anti-AIDS agents, 2: Inhibitory effects of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells". J. Nat. Prod. 53 (3): 587–95. doi:10.1021/np50069a008. PMID 1698933. http://grande.nal.usda.gov/ibids/index.php?mode2=detail&origin=ibids_references&therow=220754.
- ↑ Nobre-Junior, Helio V. et al. (2007). "Neuroprotective Actions of Tannins from Myracrodruon urundeuva on 6-Hydroxydopamine-Induced Neuronal Cell Death". Journal of Herbs, Spices & Medicinal Plants (Haworth Press) 13 (2). http://www.haworthpress.com/store/Toc_views.asp?sid=TEJBLR1GTE9P9L4KKH98M4BFKMUS112B&TOCName=J044v13n02%5FTOC&desc=Volume%3A%2013%20Issue%3A%202. Retrieved 8 November 2007.
- ↑ Souza, S. M. C. et al.; Aquino, LC; Milach Jr, AC; Bandeira, MA; Nobre, ME; Viana, GS (2006). "Antiinflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemão (Anacardiaceae) in Rodents". Phytotherapy Research (John Wiley & Sons) 21 (3): 220–225. doi:10.1002/ptr.2011. PMID 17154231.
- ↑ www.natural-specialities.com
General references
- Calvi L, Mwalongo GCJ, Mwingira BA, Riedl B, Shields JA (1995). "Characterization of Wattle-Tannin-Based Adhesives for Tanzania". Holzforchung 49 (2).
External links
|
||||||||||||||
|
||||||||||||||
|
|||||||||||||||||