Oseltamivir
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| ethyl (3R,4R,5S)-5-amino-4-acetamido-3-
(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate |
|
| Identifiers | |
| CAS number | 196618-13-0 |
| ATC code | J05AH02 |
| PubChem | CID 65028 |
| DrugBank | DB00198 |
| Chemical data | |
| Formula | C16H28N2O4 |
| Mol. mass | 312.4 g/mol |
| SMILES | eMolecules & PubChem |
| Pharmacokinetic data | |
| Bioavailability | 75% |
| Metabolism | Hepatic, to GS4071 |
| Half-life | 6–10 hours |
| Excretion | Renal (GS4071) |
| Therapeutic considerations | |
| Pregnancy cat. | B1(AU) C(US) |
| Legal status | Prescription Only (S4) (AU) POM (UK) ℞-only (US) |
| Routes | oral |
Oseltamivir (INN) (pronounced /ɒsəlˈtæmɨvɪər/) is an antiviral drug that slows the spread of influenza (flu) virus between cells in the body by stopping the virus from chemically cutting ties with its host cell—median time to symptom alleviation is reduced by 0.5–1 day[1]. The drug is sold under the trade name Tamiflu and is taken orally in capsules or as a suspension. It has been used to treat and prevent Influenzavirus A and Influenzavirus B infection in over 50 million people since 1999.
Oseltamivir is a prodrug, a (relatively) inactive chemical which is converted into its active form by metabolic process after it is taken into the body. It was the first orally active neuraminidase inhibitor commercially developed. It was developed by C.U. Kim, W. Lew and X. Chen of US based Gilead Sciences and is currently marketed by Hoffmann–La Roche (Roche). In Japan, it is marketed by Chugai Pharmaceutical Co., which is more than 50% owned by Roche.[2][3]
As of May 2010[update], the World Health Organization (WHO) reported 285 out of over 15,000 samples of the prevalent 2009 pandemic H1N1 (swine) flu tested worldwide have shown resistance to oseltamivir,[4] contrasting sharply with the 99.6% of the seasonal H1N1 flu strains tested which have resistance to oseltamivir.[5]
Contents |
Clinical usage
Mechanism of action
The prodrug Oseltamivir is itself not virally effective; however, once in the liver, it is converted by natural chemical processes, hydrolysed hepatically to its active metabolite, the free carboxylate of oseltamivir (GS4071).[6]
Oseltamivir is a neuraminidase inhibitor, serving as a competitive inhibitor towards sialic acid, found on the surface proteins of normal host cells. By blocking the activity of the viral neuraminidase enzyme, oseltamivir prevents new viral particles from being released by infected cells.[6]
Indications and dosage
Oseltamivir is marketed by Roche under the trade name Tamiflu, as capsules (containing oseltamivir phosphate 98.5 mg equivalent to oseltamivir 75 mg) and as a powder for oral suspension (oseltamivir phosphate equivalent to oseltamivir 12 mg/ml).
Oseltamivir is indicated for the treatment and prevention of infections due to influenza A and B virus.
Oseltamivir is approved for use in persons age 1 and over. There is also currently an FDA Emergency Use authorization temporarily allowing the use of Tamiflu in children less than one year old.[7] The usual adult dosage for treatment of influenza is 75 mg twice daily for 5 days, beginning within 2 days of the appearance of symptoms and with decreased doses for children and patients with renal impairment. Oseltamivir may be given as a preventive measure either during a community outbreak or following close contact with an infected individual. Standard prophylactic dosage is 75 mg once daily for patients aged 13 and older, which has been shown to be safe and effective for up to six weeks. The importance of early treatment is that the NA protein inhibition is more effective within the first 48 hours. If the virus has replicated and infected many cells the effectiveness of this medication will be severely diminished, especially over time.[6][8]
The Centers for Disease Control and Prevention (CDC) recommends physicians prioritize which patients they prescribe oseltamivir to. Specifically, people hospitalized with more severe illness, children younger than 2 years old, adults over 65, pregnant women, people with certain chronic medical or immunosuppressive conditions and adults under 19 on long-term aspirin therapy. However, they also advise that children and adults presenting with suspected flu that have symptoms of lower respiratory tract illness or clinical deterioration should also receive prompt empiric antiviral therapy, regardless of previous health or age.[9]
The standard recommended dose incompletely suppresses viral replication in at least some patients with H5N1 avian influenza, increasing the risk of viral resistance and rendering therapy less effective.[10] Accordingly, it has been suggested that higher doses and longer durations of therapy should be used for treatment of patients with the H5N1 virus.[10][11]
Clinical trials for an increased dosage began in May 2007. All avian influenza cases in Indonesia, Thailand, and Vietnam were inducted into the trial. The trial also included 100 cases of severe seasonal influenza from each of those countries and the United States. Half received the current standard dose, and half received a double dose, but for the standard length of time.[12]
Efficacy
On December 8, 2009 the Cochrane Collaboration, which reviews medical evidence, announced in a review published in the British Medical Journal that it had reversed its previous findings that the antiviral drug Tamiflu can ward off pneumonia and other serious conditions linked to influenza. They reported that an analysis of 20 studies showed Tamiflu offered mild benefits for healthy adults if taken within 24 hours of onset of symptoms, but found no clear evidence it prevented lower respiratory tract infections or other complications of influenza.[13][14] Their published finding relates only to its use in healthy adults with influenza; they say nothing about its use in patients judged to be at high risk of complications—pregnant women, children under 5, and those with underlying medical conditions, and uncertainty over its role in reducing complications in healthy adults may still leave it as a useful drug for reducing the duration of symptoms.[15]
Co-administration with probenecid
It has been suggested that co-administration of oseltamivir with probenecid could extend a limited supply of oseltamivir. Probenecid reduces renal excretion of the active metabolite of oseltamivir. One study showed that 500 mg of probenecid given every six hours doubled both the peak plasma concentration (Cmax) and the half-life of oseltamivir, increasing overall systemic exposure (AUC) by 150 percent.[16] Although the evidence for this interaction comes from a study by Roche, it was publicised only in October 2005 by a doctor who had reviewed the data.[17] Probenecid was used in similar fashion during World War II to extend limited supplies of penicillin. It is still used to increase penicillin concentrations in serious infections.
Possible side effects
Common adverse drug reactions (ADRs) associated with oseltamivir therapy (occurring in over 1% of clinical trial participants) include: nausea, vomiting, diarrhea, abdominal pain, and headache. Rare ADRs include: hepatitis and elevated liver enzymes, rash, allergic reactions including anaphylaxis, and Stevens-Johnson syndrome.[6][8]
Various other ADRs have been reported in postmarketing surveillance including: toxic epidermal necrolysis, cardiac arrhythmia, seizure, confusion, aggravation of diabetes, and haemorrhagic colitis.[6]
There are concerns that oseltamivir may cause dangerous psychological, neuropsychiatric side effects including self harm in some users. These dangerous side effects occur more commonly in children than in adults.[18] This stems from cases in Japan, where the drug is most heavily prescribed, consuming 60% of the world's production.[19] Concern has focused on teenagers, but problems have also been reported in children and adults.
In March 2007, Japan's Health Ministry warned that oseltamivir should not be given to those aged 10 to 19. The Ministry had previously decided, in May 2004, to change the literature accompanying oseltamivir to include neurological and psychological disorders as possible adverse effects, including: impaired consciousness, abnormal behavior, and hallucinations.[19]
According to Japan's Health Ministry, between 2004 and March 2007, fifteen people aged 10 to 19 have been injured or killed by jumps or falls from buildings after taking oseltamivir, and one 17-year-old died after he jumped in front of a truck.[20] A renewed investigation of the Japanese data was completed in April 2007. It found that 128 patients had been reported to behave abnormally after taking oseltamivir since 2001. Forty-three of them were under 10 years old, 57 patients were aged 10 to 19, and 28 patients were aged 20 or over. Eight people, including five teens and three adults, had died from these actions.[21][22][20]
In October 2006, Shumpei Yokota, a professor of pediatrics at Yokahama City University, released the results of research involving around 2,800 children which found no difference in the behavior between those who took oseltamivir and those who did not. Chugai Pharmaceutical Co. (which produces oseltamivir in Japan) gave Yokota's department 10 million yen (about US$105,000) over five years.[23]
To determine whether to lift the 2007 ban, a research team from the Japanese Health, Labour and Welfare Ministry studied 10,000 children under the age of 18 who had been diagnosed with influenza since 2006. The study was finalised in April 2009. Taking into account all degrees of abnormal behaviour, including minor behavioural problems such as incoherent speech, the study found that children who took oseltamivir were 54 percent more likely to exhibit abnormal behaviour than those who did not take the drug. When the team limited its analysis to children who had displayed serious abnormal behaviour that led to injury or death, it found those who had taken oseltamivir were 25 per cent more likely to behave unusually.[24]
In November 2006, the United States Food and Drug Administration (FDA) amended the warning label to include the possible side effects of delirium, hallucinations, or other related behavior.[25] This went further than the FDA's previous pronouncement, from a year before, that there was insufficient evidence to claim a causal link between oseltamivir use and the deaths of 12 Japanese children (only two were from neurological problems, although more have died since then).[26] The change to a more cautionary stance was attributed to 103 new reports that the FDA received of delirium, hallucinations and other unusual psychiatric behavior, mostly involving Japanese patients, received between August 29, 2005 and July 6, 2006. This was an increase from the 126 similar cases logged between the drug's approval in 1999 and August 2005.[27]
Roche points out that oseltamivir has been used to treat over 50 million people since 1999, and states that influenza may itself cause psychological problems.
In March 2007, the European Medicines Agency said that the benefits of oseltamivir outweighed the costs, but that it would closely monitor reports from Japan.
In April 2007, South Korea issued a safety warning against prescribing oseltamivir to teenagers except in special cases.[28]
A joint investigation by the British Medical Journal (BMJ) and British TV Channel 4 published in the BMJ on December 8, 2009 [29][30] concluded that in otherwise healthy adults they "have no confidence in claims that oseltamivir reduces the risk of complications and hospital admission in people with influenza" and believe it should not be used in routine control of seasonal influenza. There was also concern about under-reporting of side effects of the drug. In contrast, according to the BMJ, Roche has stated in media briefings that oseltamivir reduced hospital admissions by 61%; secondary complications (including bronchitis, pneumonia, and sinusitis) by 67% in otherwise healthy individuals and lower respiratory tract infections requiring antibiotics by 55%.
BMJ editor Dr. Fiona Godlee, said "claims that oseltamivir reduces complications have been a key justification for promoting the drug's widespread use. Governments around the world have spent billions of pounds on a drug that the scientific community has found itself unable to judge."
There is evidence that oseltamivir has a modest effect in reducing some minor flu symptoms and contagiousness in otherwise healthy adults by about one day, but this is probably not the main reason most doctors are prescribing the drug for their patients. This less important benefit may well be offset by the risks of the drug.
Mutagenesis, cell transformation, impairment of fertility, and carcinogenesis
Although found to be non-mutagenic in the Ames test and the mouse micronucleus test, Tamiflu tested positive in the Syrian Hamster Embryo (SHE) cell transformation test.[31][32]
Resistance
Mutations conferring resistance are single amino acid residue substitutions (His274Tyr) in the neuraminidase enzyme.[11]
2009 Pandemic H1N1 (swine) flu
As of May 2010[update], the World Health Organization (WHO) reported 285 out of over 15,000 samples of the prevalent 2009 pandemic H1N1 (swine) flu tested worldwide have shown resistance to oseltamivir.[4]
A study published in the 2009 June Issue of Nature Biotechnology also emphasized the need for augmentation of oseltamivir stockpiles with additional antiviral drugs including zanamivir (Relenza) based on an evaluation of the performance of these drugs in the scenario that the 2009 H1N1 'Swine Flu' neuraminidase (NA) were to acquire the oseltamivir-resistance mutation which is currently widespread in seasonal H1N1 strains.[33]
Seasonal H1N1
According to the CDC, oseltamivir is not very effective in the 2008 seasonal H1N1 virus anymore due to acquired resistance in 99.6% of all 2008 seasonal H1N1 strains, up from 12% in 2007-2008 flu season.[34]
H3N2
Mutant H3N2 influenza A virus isolates resistant to oseltamivir were found in 18% of a group of 50 Japanese children treated with oseltamivir.[35] Several explanations were proposed by the authors of the studies for the higher-than-expected resistance rate detected. First, children typically have a longer infection period, giving a longer time for resistance to develop. Second, Kiso et al. claim to have used more rigorous detection techniques than previous studies.[35]
As of October 3, 2009[update], none of the 264 H3N2 samples tested by the CDC have shown any signs of resistance to oseltamivir.
Influenza B
In 2007, Japanese investigators detected neuraminidase-resistant Influenza B virus strains in individuals who had not been treated with these drugs. The prevalence was 1.7%.[36] According to the CDC, as of October 3, 2009 no influenza B strains tested have shown any resistance to oseltamivir.
H5N1 avian influenza
High-level resistance has been detected in one girl suffering from H5N1 avian influenza in Vietnam. She was being treated with oseltamivir at time of detection.[37][38] de Jong et al. (2005) describe resistance development in two more Vietnamese patients suffering from H5N1, and compare their cases with six others. They suggest that the emergence of a resistant strain may be associated with a patient's clinical deterioration. They also note that the recommended dosage of oseltamivir does not always completely suppress viral replication, a situation that could favor the emergence of resistant strains. Moscona (2005) gives a good overview of the resistance issue, and says that personal stockpiles of oseltamivir could lead to under-dosage and thus the emergence of resistant strains of H5N1.[39]
Resistance is of concern in the scenario of an influenza pandemic (Wong and Yuen 2005), and may be more likely to develop in avian influenza than seasonal influenza due to the potentially longer duration of infection by novel viruses. Kiso et al. suggest that "a higher prevalence of resistant viruses should be expected" during a pandemic.[35] Resistant strains have also appeared in the EU.[40][41]
H5N1 has not yet been transmissible from person to person and is acquired by people working with or near infected poultry.
Commercial issues
The patent for oseltamivir is held by Gilead Sciences and is valid at least until 2016. Gilead licensed the exclusive rights to Roche in 1996. The drug does not enjoy patent protection in Thailand, Philippines, Indonesia and several other countries.[42] Gilead is politically well connected: Donald Rumsfeld served as chairman from 1997 until he became U.S. Secretary of Defense in 2001; former Secretary of State George Shultz and the wife of former California Governor Pete Wilson serve on the board.[43]
Oseltamivir was widely used during the H5N1 avian influenza epidemic in Southeast Asia in 2005. In response to the epidemic, various governments – including those of the United Kingdom, Canada, Israel, United States and Australia – stockpiled quantities of oseltamivir in preparation for a possible pandemic.
In late October 2005, Roche announced that it was suspending shipments to pharmacies in the United States and Canada until the North American seasonal flu outbreak began, to address concerns about private stockpiling and to preserve supplies for seasonal influenza.[44] Sales were suspended in Hong Kong as well, and on November 8, 2005, also in China. Roche said it would instead send all supplies to China's health ministry.[45]
On November 9, 2005, Vietnam became the first country to be granted permission by Roche to produce a generic version of oseltamivir.[46] The week before, Thai authorities said they would begin producing generic oseltamivir, claiming that Roche had not patented Tamiflu in Thailand.[47] The first Thai generic oseltamivir was produced in February 2006 and was to have been available to the public in July 2006.
In November 2005, U.S. President George W. Bush requested that Congress fund US$1 billion for the production and stockpile of oseltamivir, after Congress had already approved $1.8 billion for military use of the drug. Defense Secretary Rumsfeld recused himself from all government decisions regarding the drug.[48]
In December 2005, Roche also signed a sublicense for complete oseltamivir production with China's Shanghai Pharmaceuticals, and by March 2006 a sublicense had also been granted to India's Hetero.[49][50]
In late May 2006, the World Health Organization asked Roche to be ready to ship an emergency stockpile of oseltamivir to Indonesia if needed. The alert was in response to suspected human-to-human transmission within a family and was planned to last for two weeks.[51]
In December 2008, the Indian drug company, Cipla won its case in India's court system allowing it to manufacture a cheaper generic version of Tamiflu, called Antiflu. In May 2009, Cipla won approval from the World Health Organization certifying that its drug Antiflu was as effective as Tamiflu, and Antiflu is included in the World Health Organization list of prequalified medicinal products.[52]

Production shortage/shikimic acid
In early 2005, Roche announced a production shortage. (See Pandemic Fears, above). However, 2006, Roche said that production was about to reach 400-million treatment courses annually, that "capacity was well in excess of total government orders placed to date," and that "the supply shortage no longer exists." Total government orders between 2005 and 2007 were estimated to be around 200 million treatment doses. In fact, Roche CEO William Burns said that a shortage of orders could cause Roche to reduce production in the future. Roche attributes production increases in part to its agreements with 15 external contractors in 9 countries.[53][54][55]
While current demand for seasonal influenza treatment and pandemic stockpiling are being met, it is unclear what the situation would be if a pandemic actually started. Doctors are now testing a doubling of the standard dose with the hopes that it would cut H5N1 influenza virus death rate.[56] If this became the new standard, it would decrease the effective supply.
According to Roche, the major bottleneck in oseltamivir production is the availability of shikimic acid, which cannot be synthesised economically and is only effectively isolated from Chinese star anise, an ancient cooking spice; the herb is also used in Traditional Chinese Medicine. Although most autotrophic organisms produce shikimic acid, the isolation yield is low. A shortage of star anise is one of the key reasons why there is a worldwide shortage of Tamiflu (as of 2005). Star anise is grown in four provinces in China and harvested between March and May. It is also produced in Lang Son province, Vietnam. The shikimic acid is extracted from the seeds in a ten-stage process. Thirteen grams of star anise make 1.3 grams of shikimic acid, which can be made into 10 oseltamivir 75 mg capsules. Ninety percent of the harvest is already used by Roche in making oseltamivir.
Some academic experts and other drug companies are disputing the difficulty of producing shikimic acid by means other than star anise extraction. An alternative method for production of the acid involves fermentation of genetically-modified bacteria. Recently, biosynthetic pathways in Escherichia coli have been enhanced to allow the organism to accumulate enough shikimic acid to be used commercially.[57][58][59] Canadian generic drug company Apotex is attempting to modify oseletamivir to use a synthetic alternative to shikimic acid. Other potential sources of shikimic acid include the sweetgum and ginkgo trees. Quinic acid, derived from the bark of the cinchona tree, is a potential alternative base material for the production of oseltamivir. In addition, Aminoshikimic acid, biosynthesized via fermentation of genetically-modified bacteria (Guo and Frost),[60] is a very promising alternative starting material for the production of oseltamivir.
The multistep synthesis above shows that although the major bottleneck for Roche may be the availability of shikimic acid, production of oseltamivir is very involved. Increasing production volume (by Roche or others) would require construction of extensive new facilities (which may not be amenable to scaleup and, even if identical on paper, may not necessarily produce acceptable yields), and even if current facilities could handle a larger feedstock quantity, there would be a delay in production as the material makes it down the pipeline (~6 months or so). Producing large amounts of Tamiflu not only takes months to complete, but is also hazardous. Some of the steps in the synthesis require careful handling and relatively mild reaction conditions, as they involve the use of potentially explosive azide chemistry. Roche has explored ways to speed up production (Chimia 2004, 58, 621). It has developed an azide-free allylamine route from the epoxide to Tamiflu. It has also crafted routes that don’t rely on (–)-shikimic acid: a Diels-Alder-based one that uses furan and ethyl acrylate as starting materials and another that relies on catalytic hydrogenation of an isophthalic acid derivative followed by enzymatic desymmetrization. In addition, Frost and Guo at Michigan State University has developed a microbial synthesis of Aminoshikimic acid, which could reduce the need for azide chemistry if used as a starting material.[61]
Personal stockpiling
A short supply of oseltamivir prompted some individuals to stockpile the drug. Several American states issued advisories strongly discouraging this practice. Production has since caught up with current demand (see above).
In the New England Journal of Medicine, Anne Moscona (2005) argues that the use of personal stockpiles of oseltamivir could result in the administration of low dosages, allowing for the development of drug-resistant virus strains.[39] Many stockpilers will only have ten 75 mg pills (the current recommended dosage for oseltamivir), but this may be insufficient for the treatment of H5N1.[10]
Another argument against individual stockpiling is that limited drugs should be kept for more strategic deployment, that is, to hard-hit areas, to people in critical roles (e.g., healthcare and government workers), to people vulnerable to seasonal flu, or to people who actually have come down with avian influenza. Ethical arguments are sometimes made as to whether affluent people or nations should have preferred access to antiviral medications. Illegal importation might divert the drug from poorer countries where the risk of avian influenza is actually higher. A counter argument is that it is difficult to justify prohibition of individual stockpiling, when some of the same arguments are pertinent to corporate stockpiling, which is both allowed and encouraged.[53]
A third argument is that it would be difficult for home users to determine whether illegally-imported Tamiflu is counterfeit. In December 2005, 53 packages of counterfeit Tamiflu tablets were intercepted by the US Customs Service in South San Francisco. The packages were labeled "Generic Tamiflu". Roche officials know of only one instance of counterfeit Tamiflu appearing outside of the United States: incorrectly-labelled tablets found in Holland, which contained only Vitamin C and lactose.[62]
An argument in favor of individual stockpiling is that Roche is on the record as saying that without more orders, they may have to actually curtail production.[54] Individual stockpiling could bring market forces to play, maintaining production capacity and allowing the total supply on hand to be higher in case demand again outstrips production in the future, for instance, during a sudden influenza outbreak.
Veterinary use
There have been anecdotal reports of oseltamivir reducing disease severity and hospitalization time in canine parvovirus infection. The drug may limit the ability of the virus to invade the crypt cells of the small intestine and decrease gastrointestinal bacteria colonization and toxin production.[63]
Chemical synthesis
Aqueous solubility of oseltamivir in form of phosphate salt is 588 mg/ml at 25°C.[64]
The current production method features a number of reaction steps, two of which involve potentially hazardous azides. A reported azide-free Roche synthesis of tamiflu is summarised graphically below:
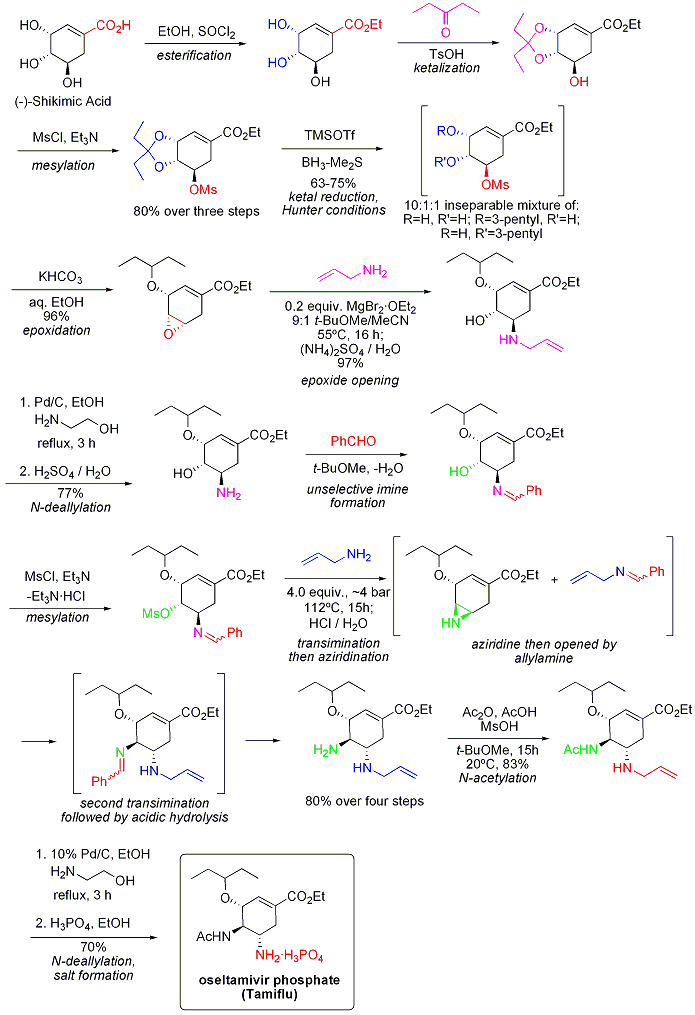
See also
- Amantadine and rimantadine - M2 inhibitors, other drugs used for influenza treatment
- Avian influenza
- H5N1
- Influenza
- Relenza / Zanamivir - another neuraminidase inhibitor
References
- ↑ Burch J, Corbett M, Stock C, et al. (September 2009). "Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis". Lancet Infect Dis 9 (9): 537–45. doi:10.1016/S1473-3099(09)70199-9. PMID 19665930. http://linkinghub.elsevier.com/retrieve/pii/S1473-3099(09)70199-9.
- ↑ "Tamiflu Approval, Review, and Labeling Information". Drugs@FDA. US FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=TAMIFLU&CFID=17854686&CFTOKEN=c50001319503aac1-21A693AF-1372-5AE1-6A3A380593AD1874. Retrieved 2009-05-08.
- ↑ Lew W, Chen X, Kim CU (June 2000). "Discovery and development of GS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor". Curr. Med. Chem. 7 (6): 663–72. PMID 10702632.
- ↑ 4.0 4.1 "Pandemic (H1N1) 2009 - update 99". World Health Organization (WHO). May 7, 2010. http://www.who.int/csr/disease/swineflu/laboratory07_05_2010/en/index.html. Retrieved May 7, 2010.
- ↑ "2008-2009 Influenza Season Week 39 ending October 3, 2009". Centers for Disease Control and Prevention (CDC). October 9, 2009. http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly39.htm. Retrieved November 20, 2009.
- ↑ 6.0 6.1 6.2 6.3 6.4 Roche Laboratories, Inc. Tamiflu product information. Last updated August 2008. (Accessed on 15 May 2009 at http://www.rocheusa.com/products/tamiflu/pi.pdf) – prescribing information document from Roche.
- ↑ http://www.cdc.gov/h1n1flu/eua/pdf/tamiflu-hcp.pdf
- ↑ 8.0 8.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ↑ http://www.cdc.gov/H1N1flu/antiviral.htm
- ↑ 10.0 10.1 10.2 PMID 16371632 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 11.0 11.1 PMID 15709056 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ "Double doses of Tamiflu for worst hit nations". IOL. 2007-03-29. http://www.int.iol.co.za/index.php?art_id=nw20070329094318857C994284. Retrieved 2009-07-29.
- ↑ Bloomberg.com http://www.bloomberg.com/apps/news?pid=20601100&sid=a9acc7qIJFdU
- ↑ BMJ http://www.bmj.com/cgi/content/abstract/339/dec07_2/b5106?ijKey=b9e57312b08cf03c1923f6a254048e5d5a08e60d&keytype2=tf_ipsecsha
- ↑ BMJ http://www.bmj.com/cgi/content/extract/339/dec10_2/b5405
- ↑ PMID 11744606 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ doi:10.1038/438006a
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Waknine, Yael (2006). "Tamiflu May Be Linked to Risk for Self-Injury and Delirium". Medscape. http://www.medscape.com/viewarticle/547783?src=mp. Retrieved 17 May 2008.
- ↑ 19.0 19.1 Parry, Richard Lloyd (March 21, 2007). "Japan issues Tamiflu warning after child deaths". The Times. http://www.timesonline.co.uk/tol/news/world/asia/article1549260.ece. Retrieved 2009-05-05.
- ↑ 20.0 20.1 "Japan to keep stockpiling Tamiflu". Sydney Morning Herald. Reuters. 2007-03-28. http://www.smh.com.au/news/World/Japan-to-keep-stockpiling-Tamiflu/2007/03/28/1174761539222.html. Retrieved 2009-07-29.
- ↑ "Japan finds 128 abnormal cases in Tamiflu probe". Forbes. AFX News Limited. 2007-05-04. http://www.forbes.com/feeds/afx/2007/04/05/afx3585482.html. Retrieved 2009-07-29.
- ↑ Russell, Sabin (2005-11-15). "Japan links Tamiflu to 2 teen suicides". San Francisco Chronicle. http://www.sfgate.com/cgi-bin/article.cgi?file=/c/a/2005/11/15/MNG29FO9K71.DTL. Retrieved 2009-07-29.
- ↑ doi:10.1038/446358a
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ "Tamiflu linked to abnormal behaviour". Sydney Morning Herald. Associated Press. 2009-04-20. http://www.smh.com.au/world/science/tamiflu-linked-to-abnormal-behaviour-20090420-ac3y.html. Retrieved 2009-07-29.
- ↑ "Flu Drug Tamiflu May Cause Odd Behavior in Children". Forbes. 2006-11-13. http://www.forbes.com/forbeslife/health/feeds/hscout/2006/11/13/hscout536084.html.
- ↑ Pediatric Advisory Committee. 2005. Pediatric safety update for Tamiflu. Rockville (MD): U.S. Food and Drug Administration.
- ↑ "FDA adds 'abnormal behavior' precaution to Tamiflu label". USA Today. Associated Press. 2006-11-14. http://www.usatoday.com/news/health/2006-11-13-tamiflu_x.htm. Retrieved 2009-07-29.
- ↑ "SKorea suspends Tamiflu use for young people - official". Forbes. AFX News Limited. 2007-04-05. http://www.forbes.com/feeds/afx/2007/04/05/afx3585631.html. Retrieved 2009-07-29.
- ↑ www.bmj.com/ -
- ↑ Examiner.com
- ↑ http://www.drugs.com/pro/tamiflu.html
- ↑ http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM183850.pdf
- ↑ Venkataramanan Soundararajan, Kannan Tharakaraman, Rahul Raman, S. Raguram, Zachary Shriver, V. Sasisekharan, Ram Sasisekharan (9 June 2009). "Extrapolating from sequence — the 2009 H1N1 'swine' influenza virus". Nature Biotechnology 27 (6): 510. doi:10.1038/nbt0609-510. PMID 19513050. http://www.nature.com/nbt/journal/v27/n6/full/nbt0609-510.html.
- ↑ http://abcnews.go.com/Health/wireStory?id=6496257
- ↑ 35.0 35.1 35.2 PMID 15337401 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 17405969 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 16228009 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ "WHO intercountry-consultation. Influenza A/H5N1 in humans in Asia. Manila, Philippines, 6–7 May 2005". World Health Organization. May 2005. http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_7/en/.
- ↑ 39.0 39.1 PMID 16371626 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Skehel JJ, Martin SR, Hay AJ, Gamblin SJ. (2008). "Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants". Nature 453 (7199): 1258. doi:10.1038/nature06956. PMID 18480754.
- ↑ Garcia-Sosa AT, Sild S, Maran U. (2008). "Design of Multi-Binding-Site Inhibitors, Ligand Efficiency, and Consensus Screening of Avian Influenza H5N1 Wild-Type Neuraminidase and of the Oseltamivir-Resistant H274Y Variant". J. Chem. Inf. Model. 48 (10): 2074–2080. doi:10.1021/ci800242z. PMID 18847186.
- ↑ Avian Flu Drugs: Patent Questions, WIPO Magazine, April 2006
- ↑ Schwartz, Nelson (Oct 31, 2005). Rumsfeld's growing stake in Tamiflu: Defense Secretary, ex-chairman of flu treatment rights holder, sees portfolio value growing. Fortune (Accessed on Nov 28, 2005 at http://money.cnn.com/2005/10/31/news/newsmakers/fortune_rumsfeld/?cnn=yes)
- ↑ "Roche to suspend Tamiflu shipments to US private sector". Forbes. AFX News Limited. 2005-10-27. http://www.forbes.com/feeds/afx/2005/10/27/afx2305441.html. Retrieved 2009-07-29.
- ↑ "Man dies of bird flu in Vietnam". Gulf Times. 2005-11-09. http://www.gulf-times.com/site/topics/article.asp?cu_no=2&item_no=59824&version=1&template_id=45&parent_id=25. Retrieved 2009-09-27.
- ↑ Tran Van Minh (2005-11-09). "Vietnam to produce generic bird-flu drug". The Washington Post. Associated Press. http://www.washingtonpost.com/wp-dyn/content/article/2005/11/09/AR2005110900384.html. Retrieved 2009-07-29.
- ↑ Le Thang Long (2005-11-10). "Roche lets Vietnam make Tamiflu". The Standard. http://www.thestandard.com.hk/news_print.asp?art_id=5316&sid=5402626. Retrieved 2009-07-29.
- ↑ Shannon Brownlee and Jeanne Lenzer (November 2009) Does the Vaccine Matter?, The Atlantic
- ↑ "China-made Tamiflu approved for production". People's Daily. 2006-06-13. http://english.people.com.cn/200606/13/eng20060613_273717.html. Retrieved 2009-07-29.
- ↑ McCoy, Michael (2006-03-20). "Making Tamiflu". Chemical & Engineering News. http://pubs.acs.org/cen/news/84/i12/8412notw7.html. Retrieved 2009-07-29.
- ↑ "WHO puts Tamiflu maker on alert after suspected human-to-human transmission". Medical News Today. 2006-05-27. http://www.medicalnewstoday.com/articles/44155.php. Retrieved 2009-07-29.
- ↑ "Cipla's anti-flu drug gets nod". Times of India. 2009-05-14. http://timesofindia.indiatimes.com/Business/India-Business/Ciplas-anti-flu-drug-gets-nod/articleshow/4526891.cms. Retrieved 2009-07-29.
- ↑ 53.0 53.1 Roche (2006-10-02). "Tamiflu readily available for 2006/2007 season". Press release. http://www.roche.com/med-cor-2006-10-02. Retrieved 2009-07-29.
- ↑ 54.0 54.1 "Roche boosts Tamiflu production; CDC cites signs of hoarding". CIDRAP. 2006-03-16. http://www.cidrap.umn.edu/cidrap/content/influenza/panflu/news/mar1606roche.html. Retrieved 2009-07-29.
- ↑ "Roche update on Tamiflu for pandemic influenza preparedness". Press release. 2006-03-16. http://www.roche.com/med-cor-2006-03-16. Retrieved 2009-07-29.
- ↑ "Doctors test double Tamiflu dose to cut H5N1 deaths". Retuers. 2007-03-28. http://www.alertnet.org/thenews/newsdesk/HKG133031.htm. Retrieved 2009-07-29.
- ↑ doi:10.1038/nrd1917
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1016/j.ymben.2003.09.001
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1002/bit.20546
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1021/ol049666e
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Frost, John. and Guo, Jiantao. (2007). Synthesis of oseltamivir carboxylates, US patent 2007/0190621 A1. http://www.google.com/patents?id=ZCeBAAAAEBAJ&dq=%22Synthesis+of+oseltamivir+carboxylates%22.
- ↑ PMID 15070787 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Macintire, Douglass K. (2006). "Treatment of Parvoviral Enteritis". Proceedings of the Western Veterinary Conference. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=wvc2006&PID=pr12457&O=VIN. Retrieved 2007-06-09.
- ↑ http://www.medicinescomplete.com/mc/ahfs/current/a399040.htm
Further reading
- Pollack, Andrew. Is Bird Flu Drug Really So Vexing? Debating the Difficulty of Tamiflu [News article]. The New York Times (Accessed on November 5, 2005 at http://www.nytimes.com/2005/11/05/business/05tamiflu.html)
- PMID 16424427 (PubMed)
Citation will be completed automatically in a few minutes.
Jump the queue or expand by hand
- doi:10.1021/jo980330q
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - doi:10.1021/jo005702l
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - doi:10.2533/000942904777677605
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - doi:10.1021/ja0616433
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - PMID 1951305 (PubMed)
Citation will be completed automatically in a few minutes.
Jump the queue or expand by hand
External links
- New: Neuraminidase inhibitors have modest effectiveness
- www.tamiflu.com – Roche's page on Tamiflu (oseltamivir)
- MedlinePlus Drug Information: oseltamivir (systemic) –Last Revised - 05/01/2009 Advice for the Patient
- Pharmasquare – Tamiflu Mode of Action – Flash animation showing the mode of action of oseltamivir
- FDA information page on oseltamivir
- Flu Drugs FAQ – U.S. National Institute of Allergy and Infectious Diseases
- Reto U. Schneider: The race to develop GS4104 - A comprehensive feature story about the development of Tamiflu published in January 2004 in NZZ-Folio, the magazine of the daily Neue Zürcher Zeitung in Switzerland (translated from German).
- Journal Response – Oseltamivir – abstracts of recent oseltamivir research
|
||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||