Serine
| Serine | |
|---|---|
 |
|
|
Serine
|
|
|
Other names
2-Amino-3-hydroxypropionic acid
|
|
| Identifiers | |
| CAS number | 302-84-1 56-45-1 (L-isomer) 312-84-5 (D-isomer) |
| PubChem | 617 |
| ChemSpider | 597 |
| EC-number | 206-130-6 |
| IUPHAR ligand | 726 |
|
SMILES
OC[C@H](N)C(=O)O
|
|
|
InChI
InChI=1/C3H7NO3/c4-2(1-5)3(6)7/h2,5H,1,4H2,(H,6,7)
|
|
| Properties[1] | |
| Molecular formula | C3H7NO3 |
| Molar mass | 105.09 g mol−1 |
| Appearance | white crystals or powder |
| Density | 1.603 g/cm3 (22 ºC) |
| Melting point |
246 ºC decomp. |
| Solubility in water | soluble |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Serine (abbreviated as Ser or S)[2] is an organic compound with the formula HO2CCH(NH2)CH2OH.
Contents |
Occurrence
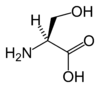
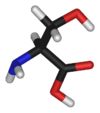
It is one of the naturally occurring proteinogenic amino acids. Its codons are UCU, UCC, UCA, UCG, AGU and AGC. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865. Its name is derived from the Latin for silk, sericum. Serine's structure was established in 1902. By virtue of the hydroxyl group, serine is classified as a polar amino acid.
Biosynthesis
The biosynthesis of serine starts with the oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate and NADH. Reductive amination of this ketone followed by hydrolysis gives serine. Serine hydroxymethyltransferase catalyzes the reversible, simultaneous conversions of L-serine to glycine (retro-aldol cleavage) and 5,6,7,8-tetrahydrofolate to 5,10-methylenetetrahydrofolate (hydrolysis).[3]
Chemical synthesis
Racemic serine can be prepared from methyl acrylate via several steps.[4] It is also naturally produced when UV light illuminates simple ices such as a combination of water, methanol, hydrogen cyanide, and ammonia, suggesting that it may be easily produced in cold regions of space. [5]
Betaines

Function
Metabolic

Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines. It is the precursor to several amino acids including glycine and cysteine, and tryptophan in bacteria. It is also the precursor to numerous of other metabolites, including sphingolipids and folate, which is the principal donor of one-carbon fragments in biosynthesis.
Structural role
Serine plays an important role in the catalytic function of many enzymes. It has been shown to occur in the active sites of chymotrypsin, trypsin, and many other enzymes. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. The unmetabolized acetylcholine cannot be recycled into the nerve for signaling. This results in depletion of acetylcholine at the neuromuscular junction, resulting in the inability to control muscles, which results in asphyxiation, and death.
As a constituent (residue) of proteins, its side chain can undergo O-linked glycosylation, which may be functionally related to diabetes. It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes. Phosphorylated serine residues are often referred to as phosphoserine. Serine proteases are a common type of protease.
Signaling
D-Serine, synthesized by serine racemase from L-serine, serves as a neuronal signal by activating NMDA receptors in the brain.[6]
See also
- Serine aggregation properties in Serine octamer clusters
References
- ↑ Weast, Robert C., ed. (1981), CRC Handbook of Chemistry and Physics (62nd ed.), Boca Raton, FL: CRC Press, p. C-512, ISBN 0-8493-0462-8.
- ↑ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem. 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ↑ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000), Principles of Biochemistry (3rd ed.), New York: W. H. Freeman, ISBN 1-57259-153-6.
- ↑ Carter, Herbert E.; West, Harold D. (1940), "dl-Serine", Org. Synth. 20: 81, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0774; Coll. Vol. 3: 774.
- ↑ Elsila, Jamie E.; Dworkin, Jason P.; Bernstein, Max P.; Martin, Mildred P.; Sandford, Scott A. (2007), "Mechanisms of Amino Acid Formation in Interstellar Ice Analogs", Astrophys. J. 660 (1): 911–18, doi:10.1086/513141.
- ↑ Mothet, Jean-Pierre; Parent, Angèle T.; Wolosker, Herman; Brady, Roscoe O., Jr.; Linden, David J.; Ferris, Christopher D.; Rogawski, Michael A.; Snyder, Solomon H. (2000), "d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor", Proc. Natl. Acad. Sci. USA 97 (9): 4926–31, doi:10.1073/pnas.97.9.4926, PMID 10781100, PMC 18334, http://www.pnas.org/cgi/pmidlookup?view=long&pmid=10781100.
|
||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||