Rosetta@home
 |
|
|---|---|
| Developer(s) | Baker laboratory, University of Washington; Rosetta Commons |
| Initial release | October 6, 2005 |
| Stable release | Rosetta Beta: 5.98, Rosetta Mini: 2.14 |
| Development status | Active |
| Operating system | Cross-platform |
| Platform | BOINC |
| License | Freeware for academic and non-profit use, commercial license available[1] |
| Website | http://boinc.bakerlab.org/rosetta |
Rosetta@home is a distributed computing project for protein structure prediction on the Berkeley Open Infrastructure for Network Computing (BOINC) platform, run by the Baker laboratory at the University of Washington. Rosetta@home aims to predict protein-protein docking and design new proteins with the help of over 81,000 volunteered computers processing at 96.680 teraFLOPS on average as of March 16, 2010.[2] Foldit, a Rosetta@Home videogame, aims to reach these goals with a crowdsourcing approach. Though much of the project is oriented towards basic research on improving the accuracy and robustness of the proteomics methods, Rosetta@home also does applied research on malaria, Alzheimer's disease and other pathologies.[3]
Like all BOINC projects, Rosetta@home uses idle computer processing resources from volunteers' computers to perform calculations on individual workunits. Completed results are sent to a central project server where they are validated and assimilated into project databases. The project is cross-platform, and runs on a wide variety of hardware configurations. Users can view the progress of their individual protein structure prediction on the Rosetta@home screensaver.
In addition to disease-related research, the Rosetta@home network serves as a testing framework for new methods in structural bioinformatics. These new methods are then used in other Rosetta-based applications, like RosettaDock and the Human Proteome Folding Project, after being sufficiently developed and proven stable on Rosetta@home's large and diverse collection of volunteer computers. Two particularly important tests for the new methods developed in Rosetta@home are the Critical Assessment of Techniques for Protein Structure Prediction (CASP) and Critical Assessment of Prediction of Interactions (CAPRI) experiments, biannual experiments which evaluate the state of the art in protein structure prediction and protein-protein docking prediction, respectively. Rosetta@home consistently ranks among the foremost docking predictors, and is one of the best tertiary structure predictors available.[4]
Contents |
Computing platform
Both the Rosetta@home application and the BOINC distributed computing platform are available for the Microsoft Windows, Linux and Macintosh platforms (BOINC also runs on several other platforms, e.g. FreeBSD).[5] Participation in Rosetta@home requires a central processing unit (CPU) with a clock speed of at least 500 MHz, 200 megabytes of free disk space, 512 megabytes of physical memory, and Internet connectivity.[6] As of May 4, 2010, the current version of the Rosetta application is 5.98, and the current version of the Rosetta Mini application is 2.14.[7] The current recommended BOINC program version is 6.2.19.[5] Standard HTTP (port 80) is used for communication between the user's BOINC client and the Rosetta@home servers at the University of Washington; HTTPS (port 443) is used during password exchange. Remote and local control of the BOINC client use port 31416 and port 1043, which might need to be specifically unblocked if they are behind a firewall.[8] Workunits containing data on individual proteins are distributed from servers located in the Baker lab at the University of Washington to volunteers' computers, which then calculate a structure prediction for the assigned protein. To avoid duplicate structure predictions on a given protein, each workunit is initialized with a random number seed. This gives each prediction a unique trajectory of descent along the protein's energy landscape.[9] Protein structure predictions from Rosetta@home are approximations of a global minimum in a given protein's energy landscape. That global minimum represents the most energetically favorable conformation of the protein, i.e. its native state.

A primary feature of the Rosetta@home graphical user interface (GUI) is a screensaver which shows a current workunit's progress during the simulated protein folding process. In the upper-left of the current screensaver, the target protein is shown adopting different shapes (conformations) in its search for the lowest energy structure. Depicted immediately to the right is the structure of the most recently accepted. On the upper right the lowest energy conformation of the current decoy is shown; below that is the true, or native, structure of the protein if it has already been determined. Three graphs are included in the screensaver. Near the middle, a graph for the accept model's free energy is displayed, which fluctuates as the accepted model changes. A graph of the accepted model's root mean square deviation (RMSD), which measures how structurally similar the accepted model is to the native model, is shown far right. On the right of the accepted energy graph and below the RMSD graph, the results from these two functions are used to produce an energy vs. RMSD plot as the model is progressively refined.[10]
Like all BOINC projects, Rosetta@home runs in the background of the user's computer using idle computer power, either at or before logging in to an account on the host operating system. Rosetta@home frees resources from the CPU as they are required by other applications so that normal computer usage is unaffected. To minimize power consumption or heat production from a computer running at sustained capacity, the maximum percentage of CPU resources that Rosetta@home is allowed to use can be specified through a user's account preferences. The times of day during which Rosetta@home is allowed to do work can also be adjusted, along with many other preferences, through a user's account settings.
Rosetta, the software that runs on the Rosetta@home network, was rewritten in C++ to allow easier development than that offered by its original version, which was written in Fortran. This new version is object-oriented, and was released on February 8, 2008.[7][11] Development of the Rosetta code is done by Rosetta Commons.[12] The software is freely licensed to the academic community and available to pharmaceutical companies for a fee.[12]
Project significance
With the proliferation of genome sequencing projects, scientists can infer the amino acid sequence, or primary structure, of many proteins that carry out functions within the cell. To better understand a protein's function and aid in rational drug design, scientists need to know the protein's three-dimensional tertiary structure.
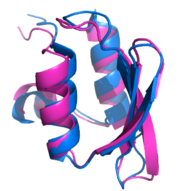
Protein 3D structures are currently determined experimentally through X-ray crystallography or nuclear magnetic resonance (NMR) spectroscopy. The process is slow (it can take weeks or even months to figure out how to crystallize a protein for the first time) and comes at high cost (around $100,000 USD per protein).[13] Unfortunately, the rate at which new sequences are discovered far exceeds the rate of structure determination – out of more than 7,400,000 protein sequences available in the NCBI non-redundant (nr) protein database, fewer than 52,000 proteins' 3D structures have been solved and deposited in the Protein Data Bank, the main repository for structural information on proteins.[14] One of the main goals of Rosetta@home is to predict protein structures with the same accuracy as existing methods, but in a way that requires significantly less time and money. Rosetta@home also develops methods to determine the structure and docking of membrane proteins (e.g., GPCRs),[15] which are exceptionally difficult to analyze with traditional techniques like X-ray crystallography and NMR spectroscopy, yet represent the majority of targets for modern drugs.
Progress in protein structure prediction is evaluated in the biannual Critical Assessment of Techniques for Protein Structure Prediction (CASP) experiment, in which researchers from around the world attempt to derive a protein's structure from the protein's amino acid sequence. High scoring groups in this sometimes competitive experiment are considered the de facto standard-bearers for what is the state of the art in protein structure prediction. Rosetta, the program on which Rosetta@home is based, has been used since CASP5 in 2002. In the 2004 CASP6 experiment, Rosetta made history by being the first to produce a close to atomic-level resolution, ab initio protein structure prediction in its submitted model for CASP target T0281.[16] Ab initio modeling is considered an especially difficult category of protein structure prediction, as it does not use information from structural homology and must rely on information from sequence homology and modeling physical interactions within the protein. Rosetta@home has been used in CASP since 2006, where it was among the top predictors in every category of structure prediction in CASP7.[17][18][19] These high quality predictions were enabled by the computing power made available by Rosetta@home volunteers.[20] Increasing computational power allows Rosetta@home to sample more regions of conformation space (the possible shapes a protein can assume), which, according to Levinthal's paradox, is predicted to increase exponentially with protein length.
Rosetta@home is also used in protein docking prediction, which determines the structure of multiple complexed proteins, or quaternary structure. This type of protein interaction affects many cellular functions, including antigen–antibody and enzyme–inhibitor binding and cellular import and export. Determining these interactions is critical for drug design. Rosetta is used in the Critical Assessment of Prediction of Interactions (CAPRI) experiment, which evaluates the state of the protein docking field similar to how CASP gauges progress in protein structure prediction. The computing power made available by Rosetta@home's project volunteers has been cited as a major factor in Rosetta's performance in CAPRI, where its docking predictions have been among the most accurate and complete.[21]
In early 2008, Rosetta was used to computationally design a protein with a function never before observed in nature.[22] This was inspired in part by the retraction of a high-profile paper from 2004 which originally described the computational design of a protein with improved enzymatic activity compared to its natural form.[23] The 2008 research paper from David Baker's group describing how the protein was made, which cited Rosetta@home for the computational resources it made available, represented an important proof of concept for this protein design method.[22] This type of protein design could have future applications in drug discovery, green chemistry, and bioremediation.[22]
In addition to basic research in predicting protein structure, docking and design, Rosetta@home is also used in immediate disease-related research.[24] Numerous minor research projects are described in David Baker's Rosetta@home journal.[25]
Alzheimer's disease
A component of the Rosetta software suite, RosettaDesign, was used to accurately predict which regions of amyloidogenic proteins were most likely to make amyloid-like fibrils.[26] By taking hexapeptides (six amino acid-long fragments) of a protein of interest and selecting the lowest energy match to a structure similar to that of a known fibril forming hexapeptide, RosettaDesign was able to identify peptides twice as likely to form fibrils as are random proteins.[27] Rosetta@home was used in the same study to predict structures for amyloid beta, a fibril-forming protein that has been postulated to cause Alzheimer's disease.[28] Preliminary but as yet unpublished results have been produced on Rosetta-designed proteins that may prevent fibrils from forming, although it is unknown whether it can prevent the disease.[29]
Anthrax
Another component of Rosetta, RosettaDock,[30][31][32] was used in conjunction with experimental methods to model interactions between three proteins—lethal factor (LF), edema factor (EF) and protective antigen (PA)—that make up anthrax toxin. The computational model accurately predicted docking between LF and PA, helping to establish which domains of the respective proteins are involved in the LF–PA complex. This insight was eventually used in research resulting in improved anthrax vaccines.[33][34]
Herpes simplex virus 1
RosettaDock was used to model docking between an antibody (immunoglobulin G) and a surface protein expressed by herpes simplex virus 1 (HSV-1) which serves to degrade the antiviral antibody. The protein complex predicted by RosettaDock closely agreed with the particularly difficult-to-obtain experimental models, leading researchers to conclude that the docking method has potential in addressing some of the problems that X-ray crystallography has with modeling protein-protein interfaces.[35]
HIV
As part of research funded by a $19.4 million dollar grant by the Bill and Melinda Gates Foundation,[36] Rosetta@home has been used in designing multiple possible vaccines for human immunodeficiency virus (HIV).[37][38]
Malaria
In research involved with the Grand Challenges in Global Health initiative,[39] Rosetta has been used to computationally design novel homing endonuclease proteins, which could eradicate Anopheles gambiae or otherwise render the mosquito unable to transmit malaria.[40] Being able to model and alter protein–DNA interactions specifically, like those of homing endonucleases, gives computational protein design methods like Rosetta an important role in gene therapy (which includes possible cancer treatments).[24][41]
Development history and branches
Originally introduced by the Baker laboratory in 1998 as an ab initio approach to structure prediction,[42] Rosetta has since branched into several development streams and distinct services. The Rosetta platform derives its name from the Rosetta Stone, as it attempts to decipher the structural "meaning" of proteins' amino acid sequences.[43] More than seven years after Rosetta's first appearance, the Rosetta@home project was released (i.e. announced as no longer beta) on October 6, 2005.[7] Many of the graduate students and other researchers involved in Rosetta's initial development have since moved to other universities and research institutions, and subsequently enhanced different parts of the Rosetta project.
RosettaDesign

RosettaDesign, a computational approach to protein design based on Rosetta, began in 2000 with a study in redesigning the folding pathway of Protein G.[44] In 2002 RosettaDesign was used to design Top7, a 93-amino acid long α/β protein that had an overall fold never before recorded in nature. This new conformation was predicted by Rosetta to within 1.2 Å RMSD of the structure determined by X-ray crystallography, representing an unusually accurate structure prediction.[45] Rosetta and RosettaDesign earned widespread recognition by being the first to design and accurately predict the structure of a novel protein of such length, as reflected by the 2002 paper describing the dual approach prompting two positive letters in the journal Science,[46][47] and being cited by more than 240 other scientific articles.[48] The visible product of that research, Top7, was featured as the Protein Data Bank's 'Molecule of the Month' in October 2006;[49] a superposition of the respective cores (residues 60-79) of its predicted and X-ray crystal structures are featured in the Rosetta@home logo.[16]
Brian Kuhlman, a former postdoctoral associate in David Baker's lab and now an assistant professor at the University of North Carolina, Chapel Hill,[50] offers RosettaDesign as an online service.[51]
RosettaDock
RosettaDock was added to the Rosetta software suite during the first CAPRI experiment in 2002 as the Baker laboratory's algorithm for protein-protein docking prediction.[52] In that experiment, RosettaDock made a high-accuracy prediction for the docking between streptococcal pyogenic exotoxin A and a T cell-receptor β-chain, and a medium accuracy prediction for a complex between porcine α-amylase and a camelid antibody. While the RosettaDock method only made two acceptably accurate predictions out of seven possible, this was enough to rank it seventh out of nineteen prediction methods in the first CAPRI assessment.[52]
Development of RosettaDock diverged into two branches for subsequent CAPRI rounds as Jeffrey Gray, who laid the groundwork for RosettaDock while at the University of Washington, continued working on the method in his new position at Johns Hopkins University. Members of the Baker laboratory further developed RosettaDock in Gray's absence. The two versions differed slightly in side-chain modeling, decoy selection and other areas.[32][53] Despite these differences, both the Baker and Gray methods performed well in the second CAPRI assessment, placing fifth and seventh respectively out of 30 predictor groups.[54] Jeffrey Gray's RosettaDock server is available as a free docking prediction service for non-commercial use.[55]
In October 2006, RosettaDock was integrated into Rosetta@home. The method used a fast, crude docking model phase using only the protein backbone. This was followed by a slow full-atom refinement phase in which the orientation of the two interacting proteins relative to each other, and side-chain interactions at the protein-protein interface, were simultaneously optimized to find the lowest energy conformation.[56] The vastly increased computational power afforded by the Rosetta@home network, in combination with revised "fold-tree" representations for backbone flexibility and loop modeling, made RosettaDock sixth out of 63 prediction groups in the third CAPRI assessment.[4][21]
Robetta
The Robetta server is an automated protein structure prediction service offered by the Baker laboratory for non-commercial ab initio and comparative modeling.[57] It has participated as an automated prediction server in the biannual CASP experiments since CASP5 in 2002, performing among the best in the automated server prediction category.[58] Robetta has since competed in CASP6 and 7, where it did better than average among both automated server and human predictor groups.[19][59][60]
In modeling protein structure as of CASP6, Robetta first searches for structural homologs using BLAST, PSI-BLAST, and 3D-Jury, then parses the target sequence into its individual domains, or independently folding units of proteins, by matching the sequence to structural families in the Pfam database. Domains with structural homologs then follow a "template-based model" (i.e., homology modeling) protocol. Here, the Baker laboratory's in-house alignment program, K*sync, produces a group of sequence homologs, and each of these is modeled by the Rosetta de novo method to produce a decoy (possible structure). The final structure prediction is selected by taking the lowest energy model as determined by a low-resolution Rosetta energy function. For domains that have no detected structural homologs, a de novo protocol is followed in which the lowest energy model from a set of generated decoys is selected as the final prediction. These domain predictions are then connected together to investigate inter-domain, tertiary-level interactions within the protein. Finally, side-chain contributions are modeled using a protocol for Monte Carlo conformational search.[61]
In CASP8, Robetta was augmented to use Rosetta's high resolution all-atom refinement method,[62] the absence of which was cited as the main cause for Robetta being less accurate than the Rosetta@home network in CASP7.[20]
Foldit
On May 9, 2008, after Rosetta@home users suggested an interactive version of the distributed computing program, the Baker lab publicly released Foldit, an online protein structure prediction game based on the Rosetta platform.[63] As of September 25, 2008, Foldit has over 59,000 registered users.[64] The game gives users a set of controls (e.g. "shake", "wiggle", "rebuild") to manipulate the backbone and amino acid side chains of the target protein into more energetically favorable conformations. Users can work on solutions individually as "soloists" or collectively as "evolvers", accruing points under either category as they improve their structure predictions.[65] Users can also individually compete with other users through a "duel" feature, in which the player with the lowest energy structure after 20 moves wins.
Comparison to similar distributed computing projects
There are several distributed computed projects which have study areas similar to those of Rosetta@home, but differ in their research approach:
Folding@home
Of all the major distributed computing projects involved in protein research, Folding@home is the only one not to use the BOINC platform.[66] Both Rosetta@home and Folding@home research protein misfolding diseases (e.g. Alzheimer's disease), but Folding@home does so much more exclusively.[67] Instead of using structure- or design-based methods to predict amyloid behavior, for example, Folding@home uses molecular dynamics to model how proteins fold (or potentially misfold, and subsequently aggregate).[68] In other words, Folding@home's strength is modeling the process of protein folding, while Rosetta@home's strength is computational protein design and prediction of protein structure and docking. The two projects also differ significantly in their computing power and host diversity. Averaging about 8.0 petaFLOPS (8000 teraFLOPS) with a host base that includes the PlayStation 3 and graphics processing units,[69] Folding@home has nearly 82 times the computing power of Rosetta@home, which averages almost 98 teraFLOPS with a host base consisting of PC-based CPUs.[2]
World Community Grid
Both Phase I and Phase II of the Human Proteome Folding Project (HPF), a subproject of World Community Grid, have used the Rosetta program to make structural and functional annotations of various genomes.[70][71] Although he now uses it to create databases for biologists, Richard Bonneau, head scientist of the Human Proteome Folding Project, was active in the original development of Rosetta at David Baker's laboratory while obtaining his PhD.[72] More information on the relationship between the HPF1, HPF2 and Rosetta@home can be found on Richard Bonneau's website.[73]
Predictor@home
Like Rosetta@home, Predictor@home specializes in protein structure prediction. Predictor@home plans to develop new areas for its distributed computing platform in protein design and docking (using the CHARMM package for molecular dynamics),[74] further likening it to Rosetta@home. While Rosetta@home uses the Rosetta program for its structure prediction, Predictor@home uses the dTASSER methodology.[75]
Other protein related distributed computing projects on BOINC include QMC@home, Docking@home, POEM@home, SIMAP, and TANPAKU. RALPH@home, the Rosetta@home alpha project which tests new application versions, work units, and updates before they move on to Rosetta@home, runs on BOINC as well.[76]
Volunteer contributions
Rosetta@home depends on computing power donated by individual project members for its research. As of November 24, 2009, over 47,000 users from 161 countries were active members of Rosetta@home, together contributing idle processor time from over 81,000 computers for a combined average performance of over 98 teraFLOPS.[2]
Users are granted BOINC credits as a measure of their contribution. The credit granted for each workunit is the number of decoys produced for that workunit multiplied by the average claimed credit for the decoys submitted by all computer hosts for that workunit. This custom system was designed to address significant differences between credit granted to users with the standard BOINC client and an optimized BOINC client, and credit differences between users running Rosetta@home on Windows and Linux operating systems.[77] The amount of credit granted per second of CPU work is lower for Rosetta@home than most other BOINC projects.[78] Despite this disadvantage to BOINC users competing for rank, Rosetta@home is fifth out of over 40 BOINC projects in terms of total credit.[79]
Rosetta@home users who predict protein structures submitted for the CASP experiment are acknowledged in scientific publications regarding their results.[20] Users who predict the lowest energy structure for a given workunit are featured on the Rosetta@home homepage as 'Predictor of the Day', along with any team of which they are a member.[80] A 'User of the Day' is chosen at random each day to be on the homepage as well from users who have made a Rosetta@home profile.[81]
References
- ↑ "Portfolio Highlight: Rosetta++ Software Suite". UW TechTransfer – Digital Ventures. http://depts.washington.edu/ventures/UW_Technology/Express_Licenses/rosetta.php. Retrieved September 7, 2008.
- ↑ 2.0 2.1 2.2 de Zutter W. "Rosetta@home: Credit overview". boincstats.com. http://boincstats.com/stats/project_graph.php?pr=rosetta. Retrieved March 16, 2010.
- ↑ "What is Rosetta@home?". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/rah_about.php. Retrieved September 7, 2008.
- ↑ 4.0 4.1 Lensink MF, Méndez R, Wodak SJ (December 2007). "Docking and scoring protein complexes: CAPRI 3rd Edition". Proteins 69 (4): 704–18. doi:10.1002/prot.21804. PMID 17918726.
- ↑ 5.0 5.1 "Download BOINC client software". BOINC. University of California. 2008. http://boinc.berkeley.edu/download_all.php. Retrieved December 1, 2008.
- ↑ "Rosetta@home: Recommended System Requirements". Rosetta@home. University of Washington. 2008. http://boinc.bakerlab.org/rosetta/rah_requirements.php. Retrieved October 7, 2008.
- ↑ 7.0 7.1 7.2 "Rosetta@home: News archive". Rosetta@home. University of Washington. 2010. http://boinc.bakerlab.org/rosetta/old_news.php. Retrieved May 4, 2010.
- ↑ "Rosetta@home: FAQ (work in progress) (message 10910)". Rosetta@home forums. University of Washington. 2006. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=669&nowrap=true#10910. Retrieved October 7, 2008.
- ↑ Kim DE (2005). "Rosetta@home: Random Seed (message 3155)". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=391&nowrap=true#3155. Retrieved October 7, 2008.
- ↑ "Rosetta@home: Quick guide to Rosetta and its graphics". Rosetta@home. University of Washington. 2007. http://boinc.bakerlab.org/rosetta/rah_graphics.php. Retrieved October 7, 2008.
- ↑ Kim DE (2008). "Rosetta@home: Problems with minirosetta version 1.+ (Message 51199)". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=3934&nowrap=true#51199. Retrieved September 7, 2008.
- ↑ 12.0 12.1 "Rosetta Commons". RosettaCommons.org. 2008. http://www.rosettacommons.org/main.html. Retrieved October 7, 2008.
- ↑ Bourne PE, Helge W, ed (2003). Structural Bioinformatics. Hoboken, NJ: Wiley-Liss. ISBN 978-0471201991. OCLC 50199108.
- ↑ "Yearly Growth of Protein Structures". RCSB Protein Data Bank. 2008. http://www.pdb.org/pdb/statistics/contentGrowthChart.do?content=molType-protein&seqid=100. Retrieved November 30, 2008.
- ↑ Baker D (2008). "Rosetta@home: David Baker's Rosetta@home journal (message 55893)". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=1177&nowrap=true#55893. Retrieved October 7, 2008.
- ↑ 16.0 16.1 "Rosetta@home: Research Overview". Rosetta@home. University of Washington. 2007. http://boinc.bakerlab.org/rosetta/rah_research.php. Retrieved October 7, 2008.
- ↑ Kopp J, Bordoli L, Battey JN, Kiefer F, Schwede T (2007). "Assessment of CASP7 predictions for template-based modeling targets". Proteins 69 Suppl 8: 38–56. doi:10.1002/prot.21753. PMID 17894352.
- ↑ Read RJ, Chavali G (2007). "Assessment of CASP7 predictions in the high accuracy template-based modeling category". Proteins 69 Suppl 8: 27–37. doi:10.1002/prot.21662. PMID 17894351.
- ↑ 19.0 19.1 Jauch R, Yeo HC, Kolatkar PR, Clarke ND (2007). "Assessment of CASP7 structure predictions for template free targets". Proteins 69 Suppl 8: 57–67. doi:10.1002/prot.21771. PMID 17894330.
- ↑ 20.0 20.1 20.2 Das R, Qian B, Raman S, et al. (2007). "Structure prediction for CASP7 targets using extensive all-atom refinement with Rosetta@home". Proteins 69 Suppl 8: 118–28. doi:10.1002/prot.21636. PMID 17894356.
- ↑ 21.0 21.1 Wang C, Schueler-Furman O, Andre I, et al. (December 2007). "RosettaDock in CAPRI rounds 6-12". Proteins 69 (4): 758–63. doi:10.1002/prot.21684. PMID 17671979.
- ↑ 22.0 22.1 22.2 Jiang L, Althoff EA, Clemente FR, et al. (March 2008). "De novo computational design of retro-aldol enzymes". Science 319 (5868): 1387–91. doi:10.1126/science.1152692. PMID 18323453.
- ↑ Hayden EC (February 13, 2008). "Protein prize up for grabs after retraction". Nature. doi:10.1038/news.2008.569.
- ↑ 24.0 24.1 "Disease Related Research". Rosetta@home. University of Washington. 2008. http://boinc.bakerlab.org/rosetta/rah_medical_relevance.php. Retrieved October 8, 2008.
- ↑ Baker D (2008). "Rosetta@home: David Baker's Rosetta@home journal". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=1177. Retrieved September 7, 2008.
- ↑ Kuhlman B, Baker D (September 2000). "Native protein sequences are close to optimal for their structures". Proceedings of the National Academy of Sciences of the United States of America 97 (19): 10383–8. doi:10.1073/pnas.97.19.10383. PMID 10984534.
- ↑ Thompson MJ, Sievers SA, Karanicolas J, Ivanova MI, Baker D, Eisenberg D (March 2006). "The 3D profile method for identifying fibril-forming segments of proteins". Proceedings of the National Academy of Sciences of the United States of America 103 (11): 4074–8. doi:10.1073/pnas.0511295103. PMID 16537487.
- ↑ Bradley P. "Rosetta@home forum: Amyloid fibril structure prediction". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=2583&sort=6. Retrieved September 7, 2008.
- ↑ Baker D. "Rosetta@home forum: Publications on R@H's Alzheimer's work? (message 54681)". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=4263&nowrap=true#54681. Retrieved October 8, 2008.
- ↑ Wang C, Schueler-Furman O, Baker D (May 2005). "Improved side-chain modeling for protein-protein docking". Protein science : a publication of the Protein Society 14 (5): 1328–39. doi:10.1110/ps.041222905. PMID 15802647.
- ↑ Gray JJ, Moughon S, Wang C, et al. (August 2003). "Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations". Journal of molecular biology 331 (1): 281–99. doi:10.1016/S0022-2836(03)00670-3. PMID 12875852.
- ↑ 32.0 32.1 Schueler-Furman O, Wang C, Baker D (August 2005). "Progress in protein-protein docking: atomic resolution predictions in the CAPRI experiment using RosettaDock with an improved treatment of side-chain flexibility". Proteins 60 (2): 187–94. doi:10.1002/prot.20556. PMID 15981249.
- ↑ Lacy DB, Lin HC, Melnyk RA, et al. (November 2005). "A model of anthrax toxin lethal factor bound to protective antigen". Proceedings of the National Academy of Sciences of the United States of America 102 (45): 16409–14. doi:10.1073/pnas.0508259102. PMID 16251269.
- ↑ Albrecht MT, Li H, Williamson ED, et al. (November 2007). "Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax". Infection and immunity 75 (11): 5425–33. doi:10.1128/IAI.00261-07. PMID 17646360.
- ↑ Sprague ER, Wang C, Baker D, Bjorkman PJ (June 2006). "Crystal structure of the HSV-1 Fc receptor bound to Fc reveals a mechanism for antibody bipolar bridging". PLoS biology 4 (6): e148. doi:10.1371/journal.pbio.0040148. PMID 16646632.
- ↑ Paulson, Tom (July 19, 2006). "Gates Foundation awards $287 million for HIV vaccine research". Seattle Post-Intelligencer. http://seattlepi.nwsource.com/local/278100_aidsvaccine19ww.html. Retrieved September 7, 2008.
- ↑ Liu Y et al. (2007). "Development of IgG1 b12 scaffolds and HIV-1 env-based outer domain immunogens capable of eliciting and detecting IgG1 b12-like antibodies" (PDF). Global HIV Vaccine Enterprise. http://www.hivvaccineenterprise.org/_dwn/Oral_Sessions.pdf. Retrieved September 28, 2008.
- ↑ Baker D. "David Baker's Rosetta@home journal archives (message 40756)". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=2431&nowrap=true#40756. Retrieved September 7, 2008.
- ↑ "Homing Endonuclease Genes: New Tools for Mosquito Population Engineering and Control". Grand Challenges in Global Health. http://www.gcgh.org/ControlInsect/Challenges/GeneticStrategy/Pages/EndonucleaseGenes.aspx. Retrieved September 7, 2008.
- ↑ Windbichler N, Papathanos PA, Catteruccia F, Ranson H, Burt A, Crisanti A (2007). "Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos". Nucleic acids research 35 (17): 5922–33. doi:10.1093/nar/gkm632. PMID 17726053.
- ↑ Ashworth J, Havranek JJ, Duarte CM, et al. (June 2006). "Computational redesign of endonuclease DNA binding and cleavage specificity". Nature 441 (7093): 656–9. doi:10.1038/nature04818. PMID 16738662.
- ↑ Simons KT, Bonneau R, Ruczinski I, Baker D (1999). "Ab initio protein structure prediction of CASP III targets using ROSETTA". Proteins Suppl 3: 171–6. doi:10.1002/(SICI)1097-0134(1999)37:3+<171::AID-PROT21>3.0.CO;2-Z. PMID 10526365.
- ↑ "Interview with David Baker". Team Picard Distributed Computing. 2006. http://www.teampicard.com/profiles/Interview.php?id=4. Retrieved December 23, 2008.
- ↑ Nauli S, Kuhlman B, Baker D (July 2001). "Computer-based redesign of a protein folding pathway". Nature structural biology 8 (7): 602–5. doi:10.1038/89638. PMID 11427890.
- ↑ Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, Baker D (November 2003). "Design of a novel globular protein fold with atomic-level accuracy". Science (New York, N.Y.) 302 (5649): 1364–8. doi:10.1126/science.1089427. PMID 14631033.
- ↑ Jones DT (November 2003). "Structural biology. Learning to speak the language of proteins". Science (New York, N.Y.) 302 (5649): 1347–8. doi:10.1126/science.1092492. PMID 14631028.
- ↑ von Grotthuss M, Wyrwicz LS, Pas J, Rychlewski L (June 2004). "Predicting protein structures accurately". Science (New York, N.Y.) 304 (5677): 1597–9; author reply 1597–9. doi:10.1126/science.304.5677.1597b. PMID 15192202.
- ↑ "Articles citing: Kuhlman et al. (2003) 'Design of a novel globular protein fold with atomic-level accuracy'". ISI Web of Science. http://www.sciencemag.org/cgi/external_ref?access_num=sci%3B302%2F5649%2F1364&link_type=ISI_Citing. Retrieved July 10, 2008.
- ↑ "October 2005 molecule of the month: Designer proteins". RCSB Protein Data Bank. http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb70_1.html. Retrieved September 7, 2008.
- ↑ "Kuhlman laboratory homepage". Kuhlman Laboratory. University of North Carolina. http://www.unc.edu/kuhlmanpg/index.htm. Retrieved September 7, 2008.
- ↑ "RosettaDesign web server". Kuhlman Laboratory. University of North Carolina. http://rosettadesign.med.unc.edu/. Retrieved September 7, 2008.
- ↑ 52.0 52.1 Gray JJ, Moughon SE, Kortemme T, et al. (July 2003). "Protein-protein docking predictions for the CAPRI experiment". Proteins 52 (1): 118–22. doi:10.1002/prot.10384. PMID 12784377.
- ↑ Daily MD, Masica D, Sivasubramanian A, Somarouthu S, Gray JJ (2005). "CAPRI rounds 3-5 reveal promising successes and future challenges for RosettaDock". Proteins 60 (2): 181–86. doi:10.1002/prot.20555. PMID 15981262. http://www3.interscience.wiley.com/cgi-bin/fulltext/110548131/HTMLSTART.
- ↑ Méndez R, Leplae R, Lensink MF, Wodak SJ (2005). "Assessment of CAPRI predictions in rounds 3-5 shows progress in docking procedures". Proteins 60 (2): 150–69. doi:10.1002/prot.20551. PMID 15981261. http://www3.interscience.wiley.com/cgi-bin/fulltext/110548130/HTMLSTART.
- ↑ "RosettaDock server". Gray laboratory. Johns Hopkins University. http://rosettadock.graylab.jhu.edu/. Retrieved September 7, 2008.
- ↑ "Protein-protein docking at Rosetta@home". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=2395. Retrieved September 7, 2008.
- ↑ "Robetta web server". Baker laboratory. University of Washington. http://robetta.bakerlab.org/. Retrieved September 7, 2008.
- ↑ Aloy P, Stark A, Hadley C, Russell RB (2003). "Predictions without templates: new folds, secondary structure, and contacts in CASP5". Proteins 53 Suppl 6: 436–56. doi:10.1002/prot.10546. PMID 14579333.
- ↑ Tress M, Ezkurdia I, Graña O, López G, Valencia A (2005). "Assessment of predictions submitted for the CASP6 comparative modeling category". Proteins 61 Suppl 7: 27–45. doi:10.1002/prot.20720. PMID 16187345.
- ↑ Battey JN, Kopp J, Bordoli L, Read RJ, Clarke ND, Schwede T (2007). "Automated server predictions in CASP7". Proteins 69 Suppl 8: 68–82. doi:10.1002/prot.21761. PMID 17894354.
- ↑ Chivian D, Kim DE, Malmström L, Schonbrun J, Rohl CA, Baker D (2005). "Prediction of CASP6 structures using automated Robetta protocols". Proteins 61 Suppl 7: 157–66. doi:10.1002/prot.20733. PMID 16187358.
- ↑ Baker D. "David Baker's Rosetta@home journal, message 52902". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=1177&nowrap=true#52902. Retrieved September 7, 2008.
- ↑ Baker D. "David Baker's Rosetta@home journal (message 52963)". Rosetta@home forums. University of Washington. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=1177&nowrap=true#52963. Retrieved September 16, 2008.
- ↑ "Foldit forums: How many users does Foldit have? Etc. (message 2)". University of Washington. http://fold.it/portal/node/444975. Retrieved September 27, 2008.
- ↑ "Foldit: Frequently Asked Questions". fold.it. University of Washington. http://fold.it/portal/info/faq. Retrieved September 19, 2008.
- ↑ "Project list - BOINC". University of California. http://boinc.berkeley.edu/wiki/Project_list. Retrieved September 8, 2008.
- ↑ "Folding@home - FAQ-Diseases". Stanford University. 2007. http://folding.stanford.edu/English/FAQ-Diseases. Retrieved September 8, 2008.
- ↑ "Folding@home - About". Stanford University. 2008. Archived from the original on July 28, 2008. http://web.archive.org/web/20080728035750/http://folding.stanford.edu/English/About. Retrieved September 8, 2008.
- ↑ "Client statistics by OS". Stanford University. http://fah-web.stanford.edu/cgi-bin/main.py?qtype=osstats. Retrieved November 24, 2009.
- ↑ Malmström L, Riffle M, Strauss CE, et al. (April 2007). "Superfamily assignments for the yeast proteome through integration of structure prediction with the gene ontology". PLoS biology 5 (4): e76. doi:10.1371/journal.pbio.0050076. PMID 17373854.
- ↑ Bonneau R (2006). "World Community Grid Message Board Posts: HPF -> HPF2 transition". Bonneau Lab, New York University. http://homepages.nyu.edu/~rb133/wcg/thread_7398.html. Retrieved September 7, 2008.
- ↑ "List of Richard Bonneau's publications". Bonneau Lab, New York University. http://homepages.nyu.edu/~rb133/papers.html. Retrieved September 7, 2008.
- ↑ Bonneau R. "World Community Grid Message Board Posts". Bonneau Lab, New York University. http://homepages.nyu.edu/~rb133/wcg/rbonneau_posts.html. Retrieved September 7, 2008.
- ↑ "Predictor@home: Developing new application areas for P@H". The Brooks Research Group. http://predictor.chem.lsa.umich.edu/scientific_update_cp.php#dpath. Retrieved September 7, 2008.
- ↑ Carrillo-Tripp M (2007). "dTASSER". The Scripps Research Institute. http://www.scripps.edu/~trippm/dtasser/. Retrieved September 7, 2008.
- ↑ "RALPH@home website". RALPH@home forums. University of Washington. http://ralph.bakerlab.org/. Retrieved September 7, 2008.
- ↑ "Rosetta@home: The new credit system explained". Rosetta@home forums. University of Washington. 2006. http://boinc.bakerlab.org/rosetta/forum_thread.php?id=2194. Retrieved October 8, 2008.
- ↑ "BOINCstats: Project Credit Comparison". boincstats.com. 2008. http://boincstats.com/stats/project_cpcs.php. Retrieved October 8, 2008.
- ↑ "Credit divided over projects". boincstats.com. http://boincstats.com/charts/chart_uk_bo_project_pie3dcredits.gif. Retrieved November 30, 2008.
- ↑ "Rosetta@home: Predictor of the day archive". Rosetta@home. University of Washington. 2008. http://boinc.bakerlab.org/rosetta/rah_old_potd.php. Retrieved October 8, 2008.
- ↑ "Rosetta@home: Protein Folding, Design, and Docking". Rosetta@home. University of Washington. 2008. http://boinc.bakerlab.org/rosetta. Retrieved October 8, 2008.
External links
- Rosetta@home Project website
- David Baker's Rosetta@home journal
- BOINC Includes platform overview, as well as a guide for installing BOINC and attaching to Rosetta@home
- BOINCstats – Rosetta@home Detailed contribution statistics
- RALPH@home Website for Rosetta@home alpha testing project
- Rosetta@home video on YouTube Overview of Rosetta@home given by David Baker and lab members
- Rosetta Commons Academic collaborative for development of the Rosetta platform
Online Rosetta services
- Robetta Protein structure prediction server
- RosettaDesign Protein design server
- RosettaDock Protein-protein docking server
|
||||||||||||||||||||