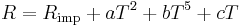Electrical resistance
| Electromagnetism | ||||||||||||
 |
||||||||||||
Electricity · Magnetism
|
||||||||||||
The electrical resistance of an object is a measure of its opposition to the passage of a steady electric current. An object of uniform cross section will have a resistance proportional to its length and inversely proportional to its cross-sectional area, and proportional to the resistivity of the material.
Discovered by Georg Ohm in 1827,[1] electrical resistance shares some conceptual parallels with the mechanical notion of friction. The SI unit of electrical resistance is the ohm (Ω). Resistance's reciprocal quantity is electrical conductance measured in siemens.
For a wide variety of materials and conditions, the electrical resistance does not depend on the amount of current through or the potential difference (voltage) across the object, meaning that the resistance R is constant for the given temperature and material. Therefore, the resistance of an object can be defined as the ratio of voltage to current, in accordance with Ohm's law:
In the case of a nonlinear conductor (not obeying Ohm's law), this ratio can change as current or voltage changes; the inverse slope of a chord to an I–V curve is sometimes referred to as a "chordal resistance" or "static resistance".[2][3]
Contents |
Resistance of a conductor
DC resistance
By a derivation of Ohm's law, the resistance R of a conductor of uniform cross section can be computed as
where
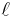 is the length of the conductor, measured in metres [m]
is the length of the conductor, measured in metres [m]
- A is the cross-sectional area of the conductor, measured in square metres [m²]
- ρ (Greek: rho) is the electrical resistivity (also called specific electrical resistance) of the material, measured in ohm-metres (Ω m). Resistivity is a measure of the material's ability to oppose electric current.
For practical reasons, any connections to a real conductor will almost certainly mean the current density is not totally uniform. However, this formula still provides a good approximation for long thin conductors such as wires.
AC resistance
If a wire conducts high-frequency alternating current, then the effective cross sectional area of the wire is reduced because of the skin effect. If several conductors are together, then due to proximity effect, the effective resistance of each is higher than if that conductor were alone.
Causes of resistance
In metals
A metal consists of a lattice of atoms, each with a shell of electrons. This is also known as a positive ionic lattice. The outer electrons are free to dissociate from their parent atoms and travel through the lattice, creating a 'sea' of electrons, making the metal a conductor. When an electrical potential difference (a voltage) is applied across the metal, the electrons drift from one end of the conductor to the other under the influence of the electric field.
Near room temperatures, the thermal motion of ions is the primary source of scattering of electrons (due to destructive interference of free electron waves on non-correlating potentials of ions), and is thus the prime cause of metal resistance. Imperfections of lattice also contribute into resistance, although their contribution in pure metals is negligible.
The larger the cross-sectional area of the conductor, the more electrons are available to carry the current, so the lower the resistance. The longer the conductor, the more scattering events occur in each electron's path through the material, so the higher the resistance. Different materials also affect the resistance.[1]
In semiconductors and insulators
In metals, the Fermi level lies in the conduction band (see Band Theory, below) giving rise to free conduction electrons. However, in semiconductors the position of the Fermi level is within the band gap, approximately half-way between the conduction band minimum and valence band maximum for intrinsic (undoped) semiconductors. This means that at 0 kelvins, there are no free conduction electrons and the resistance is infinite. However, the resistance will continue to decrease as the charge carrier density in the conduction band increases. In extrinsic (doped) semiconductors, dopant atoms increase the majority charge carrier concentration by donating electrons to the conduction band or accepting holes in the valence band. For both types of donor or acceptor atoms, increasing the dopant density leads to a reduction in the resistance. Highly doped semiconductors hence behave metallic. At very high temperatures, the contribution of thermally generated carriers will dominate over the contribution from dopant atoms and the resistance will decrease exponentially with temperature.
In ionic liquids/electrolytes
In electrolytes, electrical conduction happens not by band electrons or holes, but by full atomic species (ions) traveling, each carrying an electrical charge. The resistivity of ionic liquids varies tremendously by the concentration - while distilled water is almost an insulator, salt water is a very efficient electrical conductor. In biological membranes, currents are carried by ionic salts. Small holes in the membranes, called ion channels, are selective to specific ions and determine the membrane resistance.
Resistivity of various materials
| Material | Resistivity, 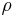 ohm-metre |
| Metals | 10–8 |
| Semiconductors | variable |
| Electrolytes | variable |
| Insulators | 1016 |
| Superconductors | 0 (exactly) |
Band theory simplified
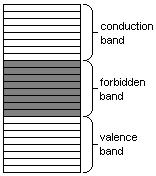
Quantum mechanics states that the energy of an electron in an atom cannot be any arbitrary value. Rather, there are fixed energy levels which the electrons can occupy, and values in between these levels are impossible. The energy levels are grouped into two bands: the valence band and the conduction band (the latter is generally above the former). Electrons in the conduction band may move freely throughout the substance in the presence of an electrical field.
In insulators and semiconductors, the atoms in the substance influence each other so that between the valence band and the conduction band there exists a forbidden band of energy levels, which the electrons cannot occupy. In order for a current to flow, a relatively large amount of energy must be furnished to an electron for it to leap across this forbidden gap and into the conduction band. Thus, even large voltages can yield relatively small currents.
Differential resistance
When the current–voltage dependence is not linear, differential resistance, incremental resistance or slope resistance is defined as the slope of the V-I graph at a particular point, thus:
This quantity is sometimes called simply resistance, although the two definitions are equivalent only for an ohmic component such as an ideal resistor. For example, a diode is a circuit element for which the resistance depends on the applied voltage or current.
If the V-I graph is not monotonic (i.e. it has a peak or a trough), the differential resistance will be negative for some values of voltage and current. This property is often known as negative resistance, although it is more correctly called negative differential resistance, since the absolute resistance V/I is still positive. An example of such an element is the tunnel diode.
Differential resistance is only useful to compare a nonlinear device with a linear source/load in some small interval; for example if it is necessary to evaluate a zener diode's voltage stability under different current values.
Temperature dependence
Near room temperature, the electric resistance of a typical metal increases linearly with rising temperature, while the electrical resistance of a typical semiconductor decreases with rising temperature. The amount of that change in resistance can be calculated using the temperature coefficient of resistivity of the material using the following formula:
where T is its temperature, T0 is a reference temperature (usually room temperature), R0 is the resistance at T0, and α is the percentage change in resistivity per unit temperature. The constant α depends only on the material being considered. The relationship stated is actually only an approximate one, the true physics being somewhat non-linear, or looking at it another way, α itself varies with temperature. For this reason it is usual to specify the temperature that α was measured at with a suffix, such as α15 and the relationship only holds in a range of temperatures around the reference.[4]
At lower temperatures (less than the Debye temperature), the resistance of a metal decreases as T5 due to the electrons scattering off of phonons. At even lower temperatures, the dominant scattering mechanism for electrons is other electrons, and the resistance decreases as T2. At some point, the impurities in the metal will dominate the behavior of the electrical resistance which causes it to saturate to a constant value. Matthiessen's Rule (first formulated by Augustus Matthiessen in the 1860s; the equation below gives its modern form) [5][6] says that all of these different behaviors can be summed up to get the total resistance as a function of temperature,
where Rimp is the temperature independent electrical resistivity due to impurities, and a, b, and c are coefficients which depend upon the metal's properties. This rule can be seen as the motivation to Heike Kamerlingh Onnes's experiments that led in 1911 to discovery of superconductivity. For details see History of superconductivity.
Intrinsic semiconductors become better conductors as the temperature increases; the electrons are bumped to the conduction energy band by thermal energy, where they flow freely and in doing so leave behind holes in the valence band which also flow freely. The electric resistance of a typical intrinsic (non doped) semiconductor decreases exponentially with the temperature:
Extrinsic (doped) semiconductors have a far more complicated temperature profile. As temperature increases starting from absolute zero they first decrease steeply in resistance as the carriers leave the donors or acceptors. After most of the donors or acceptors have lost their carriers the resistance starts to increase again slightly due to the reducing mobility of carriers (much as in a metal). At higher temperatures it will behave like intrinsic semiconductors as the carriers from the donors/acceptors become insignificant compared to the thermally generated carriers.[7]
The electric resistance of electrolytes and insulators is highly nonlinear, and case by case dependent, therefore no generalized equations are given.
Strain dependence
Just as the resistance of a conductor depends upon temperature, the resistance of a conductor depends upon strain. By placing a conductor under tension (a form of stress that leads to strain in the form of stretching of the conductor), the length of the section of conductor under tension increases and its cross-sectional area decreases. Both these effects contribute to increasing the resistance of the strained section of conductor. Under compression (strain in the opposite direction), the resistance of the strained section of conductor decreases. See the discussion on strain gauges for details about devices constructed to take advantage of this effect.
Measuring resistance
An instrument for measuring resistance is called an ohmmeter. Simple ohmmeters cannot measure low resistances accurately because the resistance of their measuring leads causes a voltage drop that interferes with the measurement, so more accurate devices use four-terminal sensing.
See also
- Electrical measurements
- Resistor
- Electrical conduction for more information about the physical mechanisms for conduction in materials.
- Ohm's law
- Voltage divider
- Voltage drop
- Current divider
- Thermal resistance
- Sheet resistance
- Electric effective resistance
- Electrical resistivity
- Electrical impedance
- Electrical reactance
- SI electromagnetism units
- Quantum Hall effect, a new standard for measuring resistance.
- Series and parallel circuits
- Proximity effect (electromagnetism)
- Skin effect
- Kondo effect
References
- ↑ David Lee. "Science Timeline". http://web.archive.org/web/20071226205343/http://www.sciencetimeline.net/1651.htm. Retrieved 2010-03-10.
- ↑ Forbes T. Brown (2006). Engineering System Dynamics. CRC Press. p. 43. ISBN 9780849396489. http://books.google.com/books?id=UzqX4j9VZWcC&pg=PA43&dq=%22chordal+resistance%22&as_brr=3&ei=Z0x0Se2yNZHGlQSpjMyvDg.
- ↑ Kenneth L. Kaiser (2004). Electromagnetic Compatibility Handbook. CRC Press. p. 13–52. ISBN 9780849320873. http://books.google.com/books?id=nZzOAsroBIEC&pg=PT1031&dq=%22static+resistance%22+%22dynamic+resistance%22+nonlinear&lr=&as_brr=3&ei=Kk50Ser1MJeOkAS9wNTwDg#PPT1031,M1.
- ↑ Ward, MR, Electrical Engineering Science, pp36–40, McGraw-Hill, 1971.
- ↑ A. Matthiessen, Rep. Brit. Ass. 32, 144 (1862)
- ↑ A. Matthiessen, Progg. Anallen, 122, 47 (1864)
- ↑ Seymour J, Physical Electronics, chapter 2, Pitman, 1972
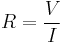


![R = R_0 [\alpha (T - T_0) + 1]\,\!](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/b5e510a563967b7edb98ee8e8ed1b280.png)