Infliximab
 |
|
|---|---|
| Monoclonal antibody | |
| Type | Whole antibody |
| Source | Chimeric (mouse/human) |
| Target | TNF |
| Identifiers | |
| CAS number | 170277-31-3 |
| ATC code | L04AB02 |
| DrugBank | BTD00004 |
| Chemical data | |
| Formula | C6428H9912N1694O1987S46 |
| Mol. mass | 144190.3 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | reticuloendothelial system |
| Half-life | 9.5 days |
| Therapeutic considerations | |
| Pregnancy cat. | B |
| Legal status | ℞-only (US) |
| Routes | IV |
Infliximab (INN; trade name Remicade) is a monoclonal antibody against TNFα. It is used to treat autoimmune diseases. Remicade is marketed by Centocor Ortho Biotech (Centocor) in the USA, Mitsubishi Tanabe Pharma in Japan, Xian Janssen in China, and Schering-Plough (now part of Merck & Co) elsewhere.[1]
Infliximab was approved by the U.S. Food and Drug Administration (FDA) for the treatment of psoriasis, Crohn's disease, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis and ulcerative colitis. Infliximab won its initial approval by the FDA for the treatment of Crohn's disease in August 1998.[2]
Infliximab works by binding to tumour necrosis factor alpha (TNFα). TNFα is a chemical messenger (cytokine) and a key part of the autoimmune reaction. Originally, it was assumed that infliximab works by blocking the action of TNFα by preventing it from binding to its receptor in the cell, and for the action of infliximab in rheumatoid arthritis. This still seems to be true. However, another TNFα-neutralizing medication, etanercept (Enbrel), is worse than a placebo in Crohn's disease and thus TNFα-neutralisation is not responsible for its powerful action in the latter disease.[3] As infliximab causes programmed cell death of TNFα-expressing activated T lymphocytes, an important cell type mediating inflammation, but Enbrel does not have this activity, now it is generally assumed that resolution of activated T cells by infliximab explains its efficacy in Crohn's disease.[4]
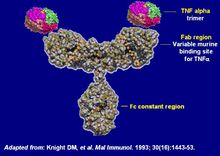
Infliximab is an artificial antibody. It was originally developed in mice, as a mouse antibody. Because humans have immune reactions to mouse proteins, it was later developed into a human (humanized) antibody. Because the antibodies were produced from one cell that was grown into a clone of identical cells, it is called a monoclonal antibody. Because it is a combination of mouse and human antibody, it is called a chimeric monoclonal antibody.
Infliximab was developed by Junming Le and Jan Vilcek at New York University School of Medicine and developed by Centocor.[5]
Infliximab can cost $19,000 to $22,000 a year per patient wholesale, according to Centocor.[6] Remicade is typically covered under major medical insurance (rather than prescription drug insurance).
Other monoclonal antibodies targeting TNFα are golimumab (Simponi), adalimumab (Humira), and certolizumab pegol (Cimzia). Etanercept also binds and inhibits the action of TNFα but is not a monoclonal antibody (it is instead a fusion of TNF-receptor and an antibody constant region).[7]
Infliximab is administered by intravenous infusion, typically at 6-8 week intervals, and at a clinic or hospital. It cannot be administered orally, because the digestive system would destroy the drug.[8]
Contents |
Pharmacology
According to product labeling, Infliximab neutralizes the biological activity of TNFα by binding with high affinity to the soluble (free floating in the blood) and transmembrane (located on the outer membranes of T cells and similar immune cells) forms of TNFα and inhibits or prevents the effective binding of TNFα with its receptors. Infliximab (Remicade) and Humira (another TNF antagonist) are in the subclass of "anti-TNF antibodies" (they are in the form of naturally occurring antibodies), and are capable of neutralizing all forms (extracellular, transmembrane, and receptor-bound) of TNF alpha.[9] Enbrel, a third TNF antagonist, is in a different subclass (receptor-construct fusion protein), and, because of its modified form, cannot neutralize receptor-bound TNFa.[10] Additionally, the anti-TNF antibodies Humira and infliximab (Remicade) have the capability of lysing cells involved in the inflammatory process, whereas the receptor fusion protein apparently lacks this capability.[11] Although the clinical significance of these differences have not been absolutely proven, they may account for the differential actions of these drugs in both efficacy and side effects.
Infliximab has high specificity for TNFα, and does not neutralize TNF beta (TNFβ, also called lymphotoxin α), an unrelated cytokine that uses different receptors from TNFα. Biological activities that are attributed to TNFα include: induction of proinflammatory cytokines such as interleukin (IL) 1 and IL 6, enhancement of leukocyte movement or migration from the blood vessels into the tissues by increasing the permeability of endothelial layer of blood vessels; and increasing the release of adhesion molecules. Infliximab prevents disease in transgenic mice (a special type of mice that are biologically engineered to produce a human form of TNFα and which are used to test the results of these drugs that might be expected in humans). These experimental mice develop arthritis as a result of their production of human TNFα, and when administered after disease onset, infliximab allows eroded joints to heal.
Infliximab in Crohn's disease
There are three phenotypes, or categories of disease presentation in Crohn's disease: stricturing disease (which causes narrowing of the bowel), penetrating disease (which causes fistulae or abnormal connections of the bowel), and inflammatory disease (which causes primarily inflammation).[12]
Fistulizing disease
Infliximab was first used for closure of fistulae in Crohn's disease in 1999. In a 94-patient phase II clinical trial, the researchers showed that Infliximab was effective in closing fistulae between the skin and bowel in 56-68% of patients.[13] A large 296-patient Phase III clinical trial called the ACCENT 2 trial, showed that infliximab was additionally beneficial in maintaining closure of fistulae, with almost two-thirds of all patients treated with the 3 initial doses infliximab (Remicade) having a fistula response after 14 weeks, and 36% of patients maintaining closure of fistulae after a year, compared with 19% who received placebo therapy. This final trial resulted in the FDA approval of the drug to treat fistulizing disease.[14]
Inflammatory disease

Infliximab has also been used in order to induce and maintain remission in inflammatory Crohn's disease. The ACCENT 1 trial was a large multicentre trial that showed that 39 to 45% of patients treated with infliximab who had an initial response to it, maintained remission after 30 weeks, compared with 21% who received placebo treatment. It also showed a mean maintenance of remission from 38 to 54 weeks compared with 21 weeks for patients who received placebo treatment.[15]
Crohn's patients have flares of their disease between periods of disease quiescence. Severe flares are usually treated with steroid medications to obtain remission, but steroids have many undesirable side effects, and therefore some gastroenterologists are now advocating for the use of infliximab as the first drug to try to get patients into remission. This has been called the top-down approach to treatment.[16]
Infliximab in ulcerative colitis
Infliximab targets TNF, thought to be more related to Th1 cytokines. Ulcerative colitis was thought to be a Th2 disease, and Infliximab of limited use. However, patients with ulcerative colitis have begun to be treated with infliximab on the basis of two large clinical trials conducted in 2005 by Paul Rutgeerts and William Sandborn. The ACT 1 and ACT 2 (Acute ulcerative Colitis Treatment) trials evaluated the utility of infliximab in ulcerative colitis and showed that 44-45% of patients treated with infliximab for a year maintained a response to the medication, compared with 21% of patients who were treated with placebo medication. At 2 months, the response was 61-69% for patients treated with infliximab, and 31% for those who were treated with placebo.[17]
Safety
According to the product labeling of infliximab, etanercept, and adalimumab, these drugs are in the class of immunosuppressants. A number of studies and reports of adverse and serious adverse reactions in patients receiving infliximab have been conducted. Risks include:
- serious and sometimes fatal blood disorders
- serious infections
- lymphoma and solid tissue cancers
- reports of serious liver injury
- reactivation of hepatitis B
- reactivation of tuberculosis
- lethal hepatosplenic T-cell lymphoma
- drug-induced lupus
- demyelinating central nervous system disorders
Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia (some fatal) have been reported with infliximab.[18] The FDA issued a warning to doctors appearing in the respective product labeling of infliximab instructing them to screen and monitor potential patients more carefully.[19] The FDA issued a warning to doctors that there is an increased risk of lymphoma and other cancers associated with the use of infliximab and other tumor necrosis factor (TNF) blockers in children and adolescents.[20]
Maintenance therapy with the drug (versus intermittent or sporadic therapy) lessens the likelihood of developing antibodies to infliximab which are known to reduce the efficacy of the drug. Combination treatment with methotrexate (an anti-folate drug which suppresses the immune system) has been shown to reduce the formation of these antibodies in patients with rheumatoid arthritis[21] and combination therapy with other immunosuppressants has been shown to reduce the likelihood of these antibodies being formed in Crohn's disease.[15] The use of immunosuppressants may not be necessary in all diseases for which infliximab is indicated, and indiscriminant use of these other immunosuppressants carry their own risks. Infliximab was studied in monotherapy (without concomitant immunosuppressants such as methotrexate or azathioprine) in psoriasis, psoriatic arthritis, and ankylosing spondylitis. Only its use in rheumatoid arthritis requires the concomitant use of methotrexate by FDA product labeling; however, the concomitant use of methotrexate in other disease states may help to reduce the body's immune response to the infliximab and increase its duration of efficacy.
Other uses
Case studies have been done into other uses of infliximab, such as to treat skin diseases. Infliximab (Remicade) was approved for treating ankylosing spondylitis, Crohn's disease, fistulizing Crohn's disease, pediatric Crohn's disease, psoriatic arthritis, psoriasis, rheumatoid arthritis, and ulcerative colitis. Infliximab is also prescribed (out of indication) for the treatment of Behçet's disease,[22] and infusions of infliximab have been used successfully in the treatment of sciatica due to slipped discs.[23]
There have been numerous case reports of the efficacy of infliximab in various inflammatory skin conditions diseases; psoriasis, in which increased TNFα has been demonstrated, is the most recent indication.[24]
Psoriatic arthritis (PsA), a chronic systemic inflammatory disorder characterized by the association of arthritis and psoriasis, follows a heterogeneous and variable clinical course. Inhibitors of TNF, such as infliximab, substantially improve the signs and symptoms of psoriasis (level 1b, grade A). Several therapies with modest efficacy have been studied in nail psoriasis. Among available agents, higher quality data are available to support the efficacy of cyclosporine and infliximab, a TNF antagonist. Based on studies in AS, the results suggest that infliximab, etanercept, and adalimumab have the potential to reduce the signs and symptoms of moderate to severely active axial involvement in PsA in patients who have had an inadequate response to NSAID (level 1a, grade A). The anti-TNF agents (infliximab and etanercept; level 1b, grade A) are more effective for the treatment of enthesitis than traditional agents. Results suggest that infliximab is effective for the treatment of dactylitis in PsA (level 1b, grade B).[25]
Availability/affordability
Like all of the TNF inhibitors, infliximab is an expensive medication, costing about US$900 for a 100 mg dose, and is covered by almost every medical insurance plan (though caps on many plans make it possible to be covered for only a subset of treatments in the course of a year). Infliximab is supplied as a sterile, white, lyophilized (freeze dried) powder[26] and must be reconstituted and administered by a health care professional, usually in a hospital or office setting.[1] For this reason it is usually covered under major medical insurance rather than prescription drug coverage.
According to the labeling, the current dosing is:
- Ankylosing spondylitis: 5 mg per kg
- Crohn's disease: 5 mg per kg
- Psoriatic arthritis: 5 mg per kg
- Rheumatoid arthritis: 3 mg per kg
- Psoriasis: 5 mg per kg
The loading regimen for all approved indications occurs at weeks 0, 2, and 6 at the above dosages.[1]
Infliximab is available from the NHS in the UK for Crohn's disease treatment provided three criteria are met.[27] Patients should have severe active Crohn's disease with a CDAI score of 300 or more, have not responded to immunomodulating drugs and corticosteroids, and for whom surgery is inappropriate.
See also
- Biological therapy for inflammatory bowel disease
External links
- Remicade Centocor Ortho Biotech
- National Rheumatoid Arthritis Society (NRAS) Information on anti-TNF drugs such as Infliximab
- Crohn's Disease: New Drug May Help When Others Fail
- Remicade Drug Label
- Full Prescribing Information and Medication Guide Centocor Ortho Biotech
References
- ↑ 1.0 1.1 1.2 Johnson & Johnson (November 6, 2007). "Remicade Becomes First Anti-TNF Biologic Therapy to Treat One Million Patients Worldwide". Press release. http://www.jnj.com/connect/NewsArchive/all-news-archive/20071106_141812. Retrieved 2009-11-14.
- ↑ "Infliximab Product Approval Information - Licensing Action". Drugs@FDA. U.S. Food and Drug Administration (FDA). http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm093327.htm. Retrieved 2009-11-14.
- ↑ Van Den Brande JM, Peppelenbosch MP, Van Deventer SJ (2002). "Treating Crohn's disease by inducing T lymphocyte apoptosis". Ann. N. Y. Acad. Sci. 973: 166–80. doi:10.1111/j.1749-6632.2002.tb04628.x. PMID 12485856.
- ↑ Van den Brande JM, Braat H, van den Brink GR, et al. (2003). "Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease". Gastroenterology 124 (7): 1774–85. doi:10.1016/S0016-5085(03)00382-2. PMID 12806611.
- ↑ Knight DM, Trinh H, Le J, et al. (1993). "Construction and initial characterization of a mouse-human chimeric anti-TNF antibody". Mol. Immunol. 30 (16): 1443–53. doi:10.1016/0161-5890(93)90106-L. PMID 8232330.
- ↑ "Priced out of pain relief; Insurers balk at high costs of promising new treatments", Victoria Colliver, San Francisco Chronicle, May 8, 2007
- ↑ Peppel, K; et al. (1991). "A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity". J. Exp. Med. 174 (6): 1483–9. doi:10.1084/jem.174.6.1483. PMID 1660525.
- ↑ Steinhilber, D; Schubert-Zsilavecz, M; Roth, HJ (2005). "Molekülstruktur und biologische Eigenschaften" (in German). Medizinische Chemie (1 ed.). Stuttgart: Deutscher Apothekerverlag. p. 5. ISBN 3-7692-3483-9.
- ↑ Choy EH, Panayi GS (2001). "Cytokine pathways and joint inflammation in rheumatoid arthritis". The New England Journal of Medicine 344 (12): 907–16. doi:10.1056/NEJM200103223441207. PMID 11259725.
- ↑ Etanercept product labeling
- ↑ Etanercept, Adalimumab and Infliximab product labeling
- ↑ Dubinsky MC, Fleshner PP. (2003). "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Curr Treat Options Gastroenterol 6 (3): 183–200. doi:10.1007/s11938-003-0001-1. PMID 12744819.
- ↑ Present D, Rutgeerts P, Targan S, Hanauer S, Mayer L, van Hogezand R, Podolsky D, Sands B, Braakman T, DeWoody K, Schaible T, van Deventer S (1999). "Infliximab for the treatment of fistulas in patients with Crohn's disease". The New England Journal of Medicine 340 (18): 1398–405. doi:10.1056/NEJM199905063401804. PMID 10228190.
- ↑ Sands B, Anderson F, Bernstein C, Chey W, Feagan B, Fedorak R, Kamm M, Korzenik J, Lashner B, Onken J, Rachmilewitz D, Rutgeerts P, Wild G, Wolf D, Marsters P, Travers S, Blank M, van Deventer S (2004). "Infliximab maintenance therapy for fistulizing Crohn's disease". The New England Journal of Medicine 350 (9): 876–85. doi:10.1056/NEJMoa030815. PMID 14985485.
- ↑ 15.0 15.1 Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P (2002). "Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial". Lancet 359 (9317): 1541–9. doi:10.1016/S0140-6736(02)08512-4. PMID 12047962.
- ↑ Hanauer SB (2003). "Crohn's disease: step up or top down therapy". Best Pract Res Clin Gastroenterol 17 (1): 131–7. doi:10.1053/bega.2003.0361. PMID 12617888.
- ↑ Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF (2005). "Infliximab for induction and maintenance therapy for ulcerative colitis". The New England Journal of Medicine 353 (23): 2462–76. doi:10.1056/NEJMoa050516. PMID 16339095.
- ↑ Remicade for Healthcare Professionals
- ↑ [1]
- ↑ "Tumor Necrosis Factor (TNF) Blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi) August 2009". MedWatch. U.S. Food and Drug Administration (FDA). August 31, 2009. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalproducts/ucm175843.htm. Retrieved 2009-11-15.
- ↑ Maini R, St Clair EW, Breedveld F, et al. (1999). "Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group". Lancet 354 (9194): 1932–9. doi:10.1016/S0140-6736(99)05246-0. PMID 10622295.
- ↑ Sfikakis PP (2002). "Behçet's disease: a new target for anti-tumour necrosis factor treatment". Ann. Rheum. Dis. 61 Suppl 2: ii51–3. PMID 12379622.
- ↑ Korhonen T, Karppinen J, Malmivaara A, et al. (2004). "Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up". Spine 29 (19): 2115–9. doi:10.1097/01.brs.0000141179.58778.6c. PMID 15454701.
- ↑ Gupta AK, Skinner AR (2004). "A review of the use of infliximab to manage cutaneous dermatoses". J Cutan Med Surg 8 (2): 77–89. doi:10.1007/s10227-004-0115-7. PMID 15685387.
- ↑ Kavanaugh AF, Ritchlin CT (2006). "Systematic review of treatments for psoriatic arthritis: an evidence based approach and basis for treatment guidelines". J. Rheumatol. 33 (7): 1417–21. PMID 16724373.
- ↑ http://www.rxlist.com/remicade-drug.htm
- ↑ "Guidance on the use of infliximab for Crohn’s disease (TA40)"
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||