RNA polymerase
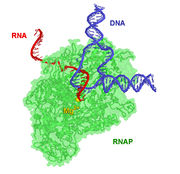
RNA polymerase (RNAP or RNApol) is an enzyme that produces RNA. In cells, RNAP is needed for constructing RNA chains from DNA genes as templates, a process called transcription. RNA polymerase enzymes are essential to life and are found in all organisms and many viruses. In chemical terms, RNAP is a nucleotidyl transferase that polymerizes ribonucleotides at the 3' end of an RNA transcript.
Contents |
History
RNAP was discovered independently by Sam Weiss, Audrey Stevens, and Jerard Hurwitz in 1960.[1] By this time the 1959 Nobel Prize in Medicine had been awarded to Severo Ochoa and Arthur Kornberg for the discovery of what was believed to be RNAP,[2] but instead turned out to be polynucleotide phosphorylase.
The 2006 Nobel Prize in Chemistry was awarded to Roger Kornberg for creating detailed molecular images of RNA polymerase during various stages of the transcription process.[3]
Control of transcription

Control of the process of gene transcription affects patterns of gene expression and, thereby, allows a cell to adapt to a changing environment, perform specialized roles within an organism, and maintain basic metabolic processes necessary for survival. Therefore, it is hardly surprising that the activity of RNAP is both long and complex and highly regulated. In Escherichia coli bacteria, more than 100 transcription factors have been identified, which modify the activity of RNAP.[4]
RNAP can initiate transcription at specific DNA sequences known as promoters. It then produces an RNA chain, which is complementary to the template DNA strand. The process of adding nucleotides to the RNA strand is known as elongation; In eukaryotes, RNAP can build chains as long as 2.4 million nucleosides (the full length of the dystrophin gene). RNAP will preferentially release its RNA transcript at specific DNA sequences encoded at the end of genes known as terminators.
Products of RNAP include:
- Messenger RNA (mRNA)—template for the synthesis of proteins by ribosomes.
- Non-coding RNA or "RNA genes"—a broad class of genes that encode RNA that is not translated into protein. The most prominent examples of RNA genes are transfer RNA (tRNA) and ribosomal RNA (rRNA), both of which are involved in the process of translation. However, since the late 1990s, many new RNA genes have been found, and thus RNA genes may play a much more significant role than previously thought.
- Transfer RNA (tRNA)—transfers specific amino acids to growing polypeptide chains at the ribosomal site of protein synthesis during translation
- Ribosomal RNA (rRNA)—a component of ribosomes
- Micro RNA—regulates gene activity
- Catalytic RNA (Ribozyme)—enzymatically active RNA molecules
RNAP accomplishes de novo synthesis. It is able to do this because specific interactions with the initiating nucleotide hold RNAP rigidly in place, facilitating chemical attack on the incoming nucleotide. Such specific interactions explain why RNAP prefers to start transcripts with ATP (followed by GTP, UTP, and then CTP). In contrast to DNA polymerase, RNAP includes helicase activity, therefore no separate enzyme is needed to unwind DNA.
RNA polymerase action
Binding and initiation
RNA Polymerase binding in prokaryotes involves the α subunit recognizing the upstream element (40 to -70 base pairs) in DNA, as well as the σ factor recognizing the -10 to -35 region. There are numerous σ factors that regulate gene expression. For example, σ70 is expressed under normal conditions and allows RNAP binding to house-keeping genes, while σ32 elicits RNAP binding to heat-shock genes.
After binding to the DNA, the RNA polymerase switches from a closed complex to an open complex. This change involves the separation of the DNA strands to form an unwound section of DNA of approximately 13 bp. Ribonucleotides are base-paired to the template DNA strand, according to Watson-Crick base-pairing interactions. Supercoiling plays an important part in polymerase activity because of the unwinding and rewinding of DNA. Because regions of DNA in front of RNAP are unwound, there is compensatory positive supercoils. Regions behind RNAP are rewound and negative supercoils are present.
Elongation
Transcription elongation involves the further addition of ribonucleotides and the change of the open complex to the transcriptional complex. RNAP cannot start forming full length transcripts because of its strong binding to the promoter. Transcription at this stage primarily results in short RNA fragments of around 9 bp in a process known as abortive transcription. Once the RNAP starts forming longer transcripts it clears the promoter. At this point, the -10 to -35 promoter region is disrupted, and the σ factor falls off RNAP. This allows the rest of the RNAP complex to move forward, as the σ factor held the RNAP complex in place.
The 17-bp transcriptional complex has an 8-bp DNA-RNA hybrid, that is, 8 base-pairs involve the RNA transcript bound to the DNA template strand. As transcription progresses, ribonucleotides are added to the 3' end of the RNA transcript and the RNAP complex moves along the DNA. Although RNAP does not seem to have the 3'exonuclease activity that characterizes the proofreading activity found in DNA polymerase, there is evidence of that RNAP will halt at mismatched base-pairs and correct it.
The addition of ribonucleotides to the RNA transcript has a very similar mechanism to DNA polymerization - it is believed that these polymerases are evolutionarily related. Aspartyl (asp) residues in the RNAP will hold onto Mg2+ ions, which will, in turn, coordinate the phosphates of the ribonucleotides. The first Mg2+ will hold onto the α-phosphate of the NTP to be added. This allows the nucleophilic attack of the 3'OH from the RNA transcript, adding an additional NTP to the chain. The second Mg2+ will hold onto the pyrophosphate of the NTP. The overall reaction equation is:
(NMP)n + NTP --> (NMP)n+1 + PPi
Termination
Termination of RNA transcription can be rho-independent or rho-dependent:
Rho-independent transcription termination is the termination of transcription without the aid of the rho protein. Transcription of a palindromic region of DNA causes the formation of a hairpin structure from the RNA transcription looping and binding upon itself. This hairpin structure is often rich in G-C base-pairs, making it more stable than the DNA-RNA hybrid itself. As a result, the 8bp DNA-RNA hybrid in the transcription complex shifts to a 4bp hybrid. These last 4 base-pairs are weak A-U base-pairs, and the entire RNA transcript will fall off of DNA.[5]
RNA polymerase in bacteria
In bacteria, the same enzyme catalyzes the synthesis of mRNA and ncRNA.
RNAP is a relatively large molecule. The core enzyme has 5 subunits (~400 kDa):
- α2: The two α subunits assemble the enzyme and bind regulatory factors. Each subunit has two domains: αCTD (C-Terminal domain) binds the UP element of the extended promoter, and αNTD (N-terminal domain) binds the rest of the polymerase. This subunit is not used on promoters without an UP element.
- β: this has the polymerase activity (catalyzes the synthesis of RNA), which includes chain initiation and elongation.
- β': binds to DNA (nonspecifically).
- ω: restores denatured RNA polymerase to its functional form in vitro. It has been observed to offer a protective/chaperone function to the β' subunit in Mycobacterium smegmatis. Now known to promote assembly.[6]
In order to bind promoter-specific regions, the core enzyme requires another subunit, sigma (σ). The sigma factor greatly reduces the affinity of RNAP for nonspecific DNA while increasing specificity for certain promoter regions, depending on the sigma factor. That way, transcription is initiated at the right region. The complete holoenzyme therefore has 6 subunits: α2ββ'σω (~480 kDa). The structure of RNAP exhibits a groove with a length of 55 Å (5.5 nm) and a diameter of 25 Å (2.5 nm). This groove fits well the 20 Å (2 nm) double strand of DNA. The 55 Å (5.5 nm) length can accept 16 nucleotides.
When not in use, RNA polymerase binds to low-affinity sites to allow rapid exchange for an active promoter site when one opens. RNA polymerase holoenzyme, therefore, does not freely float around in the cell when not in use.
Transcriptional cofactors
There are many proteins that can bind to RNAP and modify its behavior. For instance, GreA and GreB from E. coli and in most other prokaryotes can enhance the ability of RNAP to cleave the RNA template near the growing end of the chain. This cleavage can rescue a stalled polymerase molecule, and is likely involved in proofreading the occasional mistakes made by RNAP. A separate cofactor, Mfd, is involved in transcription-coupled repair, the process in which RNAP recognizes damaged bases in the DNA template and recruits enzymes to restore the DNA. Other cofactors are known to play regulatory roles; i.e. they help RNAP choose whether or not to express certain genes.
RNA polymerase in eukaryotes

Eukaryotes have several types of RNAP, characterized by the type of RNA they synthesize:
- RNA polymerase I synthesizes a pre-rRNA 45S, which matures into 28S, 18S and 5.8S rRNAs which will form the major RNA sections of the ribosome.[7]
- RNA polymerase II synthesizes precursors of mRNAs and most snRNA and microRNAs.[8] This is the most studied type, and due to the high level of control required over transcription a range of transcription factors are required for its binding to promoters.
- RNA polymerase III synthesizes tRNAs, rRNA 5S and other small RNAs found in the nucleus and cytosol.[9]
- RNA polymerase IV synthesizes siRNA in plants.[10]
- RNA polymerase V synthesizes RNAs involved in siRNA-directed heterochromatin formation in plants.[11]
There are other RNA polymerase types in mitochondria and chloroplasts. And there are RNA-dependent RNA polymerases involved in RNA interference.[12]
RNA polymerase in archaea
Archaea have a single RNAP that is closely related to the three main eukaryotic polymerases (Pol I,II,III). Thus, it has been speculated that the archaeal polymerase resembles the ancestor of the specialized eukaryotic polymerases.[13]
RNA polymerase in viruses

Many viruses also encode for RNAP. Perhaps the most widely studied viral RNAP is found in bacteriophage T7. The single-subunit T7 RNA polymerase is related to that found in mitochondria and chloroplasts, and shares considerable homology to DNA polymerase.[14] It is believed that most viral polymerases therefore evolved from DNA polymerase and are not directly related to the multi-subunit polymerases described above.
The viral polymerases are diverse, and include some forms that can use RNA as a template instead of DNA. This occurs in negative strand RNA viruses and dsRNA viruses, both of which exist for a portion of their life cycle as double-stranded RNA. However, some positive strand RNA viruses, such as polio, also contain these RNA-dependent RNA polymerases.[15]
RNA polymerase purification
RNA polymerase can be isolated in the following ways:
- By a phosphocellulose column.[16]
- By glycerol gradient centrifugation.[17]
- By a DNA column.
- By an Ion exchange column.[18]
And also combinations of the above techniques.
See also
- DNA polymerase
- T7 RNA polymerase
- RNA polymerase I
- RNA polymerase II
- RNA polymerase III
- Alpha-amanitin
References
- ↑ Jerard Hurwitz (December 2005). "The Discovery of RNA Polymerase". Journal of Biological Chemistry 280 (52): 42477–85. doi:10.1074/jbc.X500006200. PMID 16230341.
- ↑ Nobel Prize 1959
- ↑ Nobel Prize in Chemistry 2006
- ↑ Akira Ishihama (2000). Functional modulation of Escherichia coli RNA polymerase. 54. pp. 499–518. doi:10.1162/artl.2006.12.4.513. PMID 11018136.
- ↑ Farnham PJ; Platt T. (February 1981). "Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro". Nucleic Acids Res. 9 (3): 563–77. doi:10.1093/nar/9.3.563. PMID 7012794.
- ↑ Murray, Robert K.; Harper's illustrated biochemistry; McGraw-Hill Professional, 2003 ISBN 0-071-38901-6
- ↑ Grummt I. (1999). "Regulation of mammalian ribosomal gene transcription by RNA polymerase I.". Prog Nucleic Acid Res Mol Biol. 62: 109–54. doi:10.1016/S0079-6603(08)60506-1. PMID 9932453.
- ↑ Lee Y; Kim M; Han J; Yeom KH; Lee S; Baek SH; Kim VN. (October 2004). "MicroRNA genes are transcribed by RNA polymerase II". EMBO J. 23 (20): 4051–60. doi:10.1038/sj.emboj.7600385. PMID 15372072.
- ↑ Willis IM. (February 1993). "RNA polymerase III. Genes, factors and transcriptional specificity". Eur J Biochem. 212 (1): 1–11. doi:10.1111/j.1432-1033.1993.tb17626.x. PMID 8444147.
- ↑ Herr AJ, Jensen MB, Dalmay T, Baulcombe DC (2005). "RNA polymerase IV directs silencing of endogenous DNA". Science 308 (5718): 118–20. doi:10.1126/science.1106910. PMID 15692015.
- ↑ Wierzbicki AT, Ream TS, Haag JR, Pikaard CS (May 2009). "RNA polymerase V transcription guides ARGONAUTE4 to chromatin". Nat. Genet. 41 (5): 630–4. doi:10.1038/ng.365. PMID 19377477.
- ↑ Makeyev EV, Bamford DH (December 2002). "Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes". Mol. Cell 10 (6): 1417–27. doi:10.1016/S1097-2765(02)00780-3. PMID 12504016. http://linkinghub.elsevier.com/retrieve/pii/S1097276502007803.
- ↑ D Langer, J Hain, P Thuriaux and W Zillig (1995) Transcription in Archaea: Similarity to that in Eucarya PNAS 92 5768-5772
- ↑ Hedtke et al. (1997) Mitochondrial and chloroplast phage-type RNA polymerases in Arabidopsis. Science 227 809-811
- ↑ Paul Ahlquist (2002) RNA-Dependent RNA Polymerases, Viruses, and RNA Silencing. Science 296 1270-1273
- ↑ Kelly JL; Lehman IR. (August 1986). "Yeast mitochondrial RNA polymerase. Purification and properties of the catalytic subunit.". J Biol Chem. 261 (22): 10340–7. PMID 3525543.
- ↑ Honda A et al. (April 1990). "Purification and molecular structure of RNA polymerase from influenza virus A/PR8.". J Biochem (Tokyo) 107 (4): 624–8. PMID 2358436.
- ↑ Hager et al. (1990) Use of Mono Q High-Resolution Ion-Exchange Chromatography To Obtain Highly Pure and Active Escherichia coli RNA Polymerase Biochemistry 29 7890-7894
External links
- DNAi - DNA Interactive, including information and Flash clips on RNA Polymerase.
- MeSH RNA+Polymerase
- EC 2.7.7.6
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||