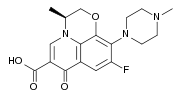
Quinolone
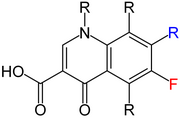
The quinolones are a family of synthetic broad-spectrum antibiotics. The term quinolone(s) refers to potent synthetic chemotherapeutic antibacterials,[1][2].
The first generation of the quinolones begins with the introduction of nalidixic acid in 1962 for treatment of urinary tract infections in humans.[3] Nalidixic acid was discovered by George Lesher and coworkers in a distillate during an attempt at chloroquine synthesis.[4]
They prevent bacterial DNA from unwinding and duplicating.[5] (See Mechanism of Action later.)
Quinolones in comparison to other antibiotic classes have the highest risk of causing colonization with MRSA and Clostridium difficile. A general avoidance of fluoroquinolones is recommended based on the available evidence and clinical guidelines.[6][7][8]. The majority of quinolones in clinical use belong to the subset of fluoroquinolones, which have a fluorine atom attached to the central ring system, typically at the 6-position or C-7 position.
Contents |
History
Nalidixic acid is considered to be the predecessor of all members of the quinolone family, including the second, third and fourth generations commonly known as fluoroquinolones. This first generation also included other quinolone drugs such as pipemidic acid, oxolinic acid, and cinoxacin, which were introduced in the 1970s. They proved to be only marginal improvements over nalidixic acid.[9] Though it is generally accepted that nalidixic acid is to be considered the first quinolone drug, this has been disputed over the years by a few researchers that believe that chloroquine, from which nalidixic acid is derived, is to be considered the first quinolone drug, rather than nalidixic acid.
Since the introduction of nalidixic acid in 1962, more than 10,000 analogs have been synthesized, but only a handful have found their way into clinical practice.[10] The fluoroquinolone drugs are the most toxic and dangerous antibiotics in clinical practice today.
Indications
It continues to be debatable as to whether or not the effectiveness of fluoroquinolones for the treatment of respiratory disorders is similar to that of other antibiotic classes.[11][12]
Fluoroquinolone use for pneumonia is increasing, and with it so is bacterial resistance to fluoroquinolones. The majority of the prescribing of fluoroquinolones is inappropriate, with less than 4 percent of people prescribed quinolones being appropriate according to clinical guidelines. Clinical guidelines in Canada recommend fluoroquinolones only for outpatient treatment of pneumonia in a small number of patients, such as those with certain co-morbid conditions, e.g., patients with a history of COPD, or those with recent use of antibiotics.[13] For severe forms of community-acquired pneumonia, the fluoroquinolones are associated with improved treatment rates, but with no differences found in mortality between other antibiotic classes.[14]
Fluoroquinolones are not recommended as first-line antibiotics for acute sinusitis, as this condition is usually self-limiting, and the risks outweigh the benefits in comparison to other antibiotic classes.[12][15]
Antibiotics including fluoroquinolones can be effective in some cases of bronchitis. However, only about 5-10% of bronchitis cases are caused by a bacterial infection; most cases of bronchitis are caused by a viral infection and are self-limiting and resolve themselves in a few weeks. It has been recommended that antibiotics are limited in most cases to those whose symptoms fail to resolve on their own.[16]
Fluoroquinolones are often used for genitourinary infections; in general they are recommended only after other antibiotic regimens have failed. However, for serious acute cases of pyelonephritis or bacterial prostatitis where the patient may need to be hospitalised, fluoroquinolones are recommended as first-line therapy.[17] Prostatitis has been termed "the waste basket of clinical ignorance" by prominent Stanford University urologist Dr. Thomas Stamey. Campbell's Urology, the urologist's most authoritative reference text, identifies only about 5% of all patients with prostatitis as having bacterial prostatitis, which can be "cured" at least in the short term by antibiotics. In other words, 95% of men with prostatitis have little hope for a cure with antibiotics alone, since they do not actually have any identifiable bacterial infection.[18]
The American Thoracic Society recommends that fluoroquinolones are not used as a first-line agent, instead recommending macrolide or doxycycline as first-line agents. The Drug-Resistant Streptococcus pneumoniae Working Group recommends that fluoroquinolones be used only after other antibiotic classes have been tried and failed, or in those with demonstrated drug-resistant Streptococcus pneumoniae. The Centers for Disease Control are concerned that fluoroquinolones are being used as a "one-size-fits-all" treatment unnecessarily by doctors without considering suitability and differences due to age and other risk factors. Effective interventions have been recommended to reduce the excessive fluoroquinolone prescribing in the United States.[19]
Adverse effects
In general, fluoroquinolones are well tolerated, with most side-effects being mild to moderate. On occasion, serious adverse effects occur.[20][21] Some of the serious adverse effects that occur more commonly with fluoroquinolones than with other antibiotic drug classes include CNS and tendon toxicity.[22][23] The currently marketed quinolones have safety profiles similar to that of other antimicrobial classes.[22] Fluoroquinolones are sometimes associated with an QTc interval prolongation and cardiac arrhythmias,[24] convulsions, tendon rupture, torsade de pointes and hypoglycemia.[25]
These adverse reactions are a class effect of all quinolones; however, certain quinolones are more strongly associated with increased toxicity to certain organs. For example, moxifloxacin carries a higher risk of QTc prolongation,[26] and gatifloxacin has been most frequently linked to disturbed blood sugar levels, although all quinolones carry these risks.[27][28] Some quinolones were withdrawn from the market because of these adverse events (for example, sparfloxacin was associated with phototoxicity and QTc prolongation, thrombocytopenia and nephritis were seen with tosufloxacin, and hepatotoxicity with trovafloxacin).[29] Simultaneous use of corticosteroids is present in almost one-third of quinolone-associated tendon rupture.[30] The risk of adverse events is further increased if the dosage is not properly adjusted, for example if there is renal insufficiency.[27]
The serious events may occur during therapeutic use at therapeutic dose levels or with acute overdose. At therapeutic doses they include: central nervous system toxicity, cardiovascular toxicity, tendon / articular toxicity, and, rarely, hepatic toxicity.[31] Caution is required in patients with liver disease.[32] Events that may occur in acute overdose are rare, and include renal failure and seizure.[31] Susceptible groups of patients, such as children and the elderly, are at greater risk of adverse reactions during therapeutic use.[22][23] Adverse reactions may manifest during, as well as after fluoroquinolone therapy has been completed.[33]
Fluoroquinolones are considered high-risk antibiotics for the development of Clostridium difficile and MRSA infections.[6][34] A previously rare strain of C. difficile that produces a more severe disease with increased levels of toxins is becoming epidemic, and may be connected to the use of fluoroquinolones.[35] Fluoroquinolones are more strongly associated with C. difficile infections than other antibiotics, including clindamycin, third-generation cephalosporins, and beta lactamase inhibitors. One study found that fluoroquinolones were responsible for 55% of C. difficile infections.[36] The European Center for Disease Prevention and Control recommends that fluoroquinolones and the antibiotic clindamycin should be avoided in clinical practice due to their high association with Clostridium difficile, a potentially life-threatening super-infection.[7]
The central nervous system is an important target for fluoroquinolone-mediated neurotoxicity. Adverse event reporting in Italy by doctors showed fluoroquinolones among the top 3 prescribed drugs for causing adverse neurological and psychiatric effects. These neuropsychiatric effects included tremor, confusion, anxiety, insomnia, agitation, and, in severe cases, psychosis. Moxifloxacin came out worst among the quinolones for causing CNS toxicity.[37] Some support and patient advocacy groups refer to these adverse events as "fluoroquinolone toxicity". Some people from these groups claim to have suffered serious long-term harm to their health from using fluoroquinolones. This has led to a class-action lawsuit by people harmed by the use of fluoroquinolones as well as action by the consumer advocate group Public Citizen.[38][39] Partly as a result of the efforts of Public Citizen, the FDA ordered black box warnings on all fluoroquinolones, advising consumers of the possible toxic effects of fluoroquinolones on tendons.[40]
Contraindications
Quinolones are contraindicated if a patient has epilepsy, QT prolongation, pre-existing CNS lesions, central nervous system inflammation or those who have suffered a stroke.[20] There are safety concerns of fluoroquinolone use during pregnancy and, as a result, are contraindicated except for when no other safe alternative antibiotic exists.[41] They are also contraindicated in children due to the risks of damage to the muscoskeletal system.[42] Their use in children is not absolutely contraindicated, however. For certain severe infections where other antibiotics are not an option, their use can be justified.[43] Quinolones should also not be given to people with a known hypersensitivity to the drug.[44][45]
Boxed warnings
U.S. Boxed Warning: Increased risk of developing tendonitis and tendon rupture in patients of all ages taking fluoroquinolones for systemic use. This risk is further increased in individuals over 60 years of age, taking corticosteroid drugs, and have received kidney, heart, or lung transplants.
Musculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to nalidixic acid.[46] Rheumatic disease after use of a fluoroquinolone (norfloxacin) was first reported eleven years later.[47] In response to a 1995 letter published in the New England Journal of Medicine, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling [package insert] for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."[48]
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen filed a petition with the FDA, prompting the agency to act.[49] Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.[50]
Nine years later, in 2005, the Illinois Attorney General filed a second petition with the FDA again seeking black box warnings and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter.[51][52][53] In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for black box warnings by filing a third petition requesting such changes be made.[51][54] When the FDA failed to respond to these two petitions as required by law, Public Citizen, in January 2008, filed suit to compel the FDA to respond to their 2006 petition.[55][56] On July 7, 2008 the FDA requested that the makers of systemic-use fluoroquinolones add a boxed warning regarding spontaneous tendon ruptures, and to develop a Medication Guide for patients.[57] The package inserts for ciprofloxacin, Avelox (moxifloxacin), Proquin XR, Factive (gemifloxacin), Floxin (ofloxacin), Noroxin (norfloxacin) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings.[58] Bayer, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes.[59] Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November.[60] through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals.
A review of the FDA website indicates that the majority of the generic versions of the fluoroquinolones have not been updated to include this Boxed Warning as of September 2009. In addition, there are numerous reports that claim that this information has not been disseminated to the pharmacist, the name brand products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmicist or physician for distribution.
Pharmacology
The basic pharmacophore, or active structure, of the fluoroquinolone class is based upon the quinoline ring system.[61] The addition of the fluorine atom at C6 is what distinguishes the successive-generation fluoroquinolones from the first-generation quinolones. It has since been demonstrated that the addition of the C6 fluorine atom is not a necessary requirement for the antibacterial activity of this class (circa 1997).[62]
Various substitutions made to the quinoline ring resulted in the development of numerous fluoroquinolone drugs available today. Each substitution is associated with a number of specific adverse reactions, as well as increased activity against bacterial infections,[10] whereas the quinoline ring, in and of itself, has been associated with severe and even fatal adverse reactions.[63]
Mechanism of action
Quinolones and fluoroquinolones are chemotherapeutic bactericidal drugs, eradicating bacteria by interfering with DNA replication. The other antibiotics used today, (e.g., tetracyclines, lincomycin, erythromycin, and chloramphenicol) do not interact with components of eukaryotic ribosomal particles and, thus, have not been shown to be toxic to eukaryotes,[64] as opposed to the fluoroquinolone class of drugs. Other drugs used to treat bacterial infections, such as penicillins and cephalosporins, inhibit cell wall biosynthesis, thereby causing bacterial cell death, as opposed to the interference with DNA replication as seen within the fluoroquinolone class of drugs.
Quinolones inhibit the bacterial DNA gyrase or the topoisomerase IV enzyme, thereby inhibiting DNA replication and transcription. Recent evidence has shown that topoisomerase II is also a target for a variety of quinolone-based drugs. Thus far, most of the compounds that show high activity against the eukaryotic type II enzyme contain aromatic substituents at their C-7 positions.[65]
Quinolones can enter cells easily via porins and, therefore, are often used to treat intracellular pathogens such as Legionella pneumophila and Mycoplasma pneumoniae. For many Gram-negative bacteria, DNA gyrase is the target, whereas topoisomerase IV is the target for many Gram-positive bacteria. It is believed that eukaryotic cells do not contain DNA gyrase or topoisomerase IV. However, there is debate concerning whether the quinolones still have such an adverse effect on the DNA of healthy cells, in the manner described above, hence contributing to their adverse safety profile. This class has been shown to damage mitochondrial DNA.[66][67][68][69][70][71][72][73]
Interactions
Theophylline, nonsteroidal anti-inflammatory drugs and corticosteroids enhance the toxicity of fluoroquinolones.[74][75][76]
Products containing multivalent cations, such as aluminum- or magnesium-containing antacids and products containing calcium, iron, or zinc, invariably result in marked reduction of oral absorption of fluoroquinolones.[77]
Other drugs that interact with fluoroquinolones include antacids, Sucralfate, Probenecid, Cimetidine, Warfarin, antiviral agents, Phenytoin, Cyclosporine, Rifampin, Pyrazinamide, and Cycloserine.[76]
Many fluoroquinolones, especially ciprofloxacin, inhibit the cytochrome P450 isoform CYP1A2.[78] This inhibition causes an increased level of, for example, antidepressants such as amitriptyline and imipramine, clozapine (an atypical antipsychotic), caffeine, Olanzapine (an atypical antipsychotic), Ropivacaine (a local anaesthetic), Theophylline (a xanthine), and Zolmitriptan (a serotonin receptor agonist).[78]
Antibiotic misuse and bacterial resistance
Resistance to quinolones can evolve rapidly, even during a course of treatment. Numerous pathogens, including Staphylococcus aureus, enterococci, and Streptococcus pyogenes now exhibit resistance worldwide.[79] Widespread veterinary usage of quinolones, in particular in Europe, has been implicated.[80]
Fluoroquinolones have been recommended to be reserved for the use in patients that are seriously ill and may soon require immediate hospitalization.[81] Though considered to be a very important and necessary drugs required to treat severe and life-threatening bacterial infections, the associated antibiotic misuse remains unchecked, which has contributed to the problem of bacterial resistance. The overuse of antibiotics such as happens with children suffering from otitis media has given rise to a breed of super-bacteria that are resistant to antibiotics entirely.[82]
For example, the use of the fluoroquinolones had increased threefold in an emergency room environment in the United States between 1995 and 2002, while the use of safer alternatives, such as macrolides, declined significantly.[19][83] Fluoroquinolones had become the most commonly prescribed class of antibiotics to adults in 2002. Nearly half (42%) of these prescriptions were for conditions not approved by the FDA, such as acute bronchitis, otitis media, and acute upper respiratory tract infection, according to a study that was supported in part by the Agency for Healthcare Research and Quality.[83][84] In addition, they are commonly prescribed for medical conditions, such as acute respiratory illness, that are usually caused by viral infections.[85]
Within a recent study concerning the proper use of this class in the emergency room, it was revealed that 99% of these prescriptions were in error. Out of the one hundred total patients studied, eighty-one received a fluoroquinolone for an inappropriate indication. Out of these cases, forty-three (53%) were judged to be inappropriate because another agent was considered first line, twenty-seven (33%) because there was no evidence of a bacterial infection to begin with (based on the documented evaluation), and eleven (14%) because of the need for such therapy was questionable. Out of the nineteen patients who received a fluoroquinolone for an appropriate indication, only one patient out of one hundred received both the correct dose and duration of therapy.[86]
There are three known mechanisms of resistance.[87] Some types of efflux pumps can act to decrease intracellular quinolone concentration. In Gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drugs' effectiveness.
Social and economic impact
Increased hospitalizations attributed to adverse drug reactions alone account for billions of dollars each year within the US healthcare system. Severe reactions do occur with the fluoroquinolone class and can add significantly to the cost of care. Antibacterial adverse effects account for nearly 25% of all adverse drug reactions among hospitalized patients.[88]
Adverse effects of fluoroquinolones can lead to patients attending hospital emergency rooms. Many of the important adverse effects of fluoroquinolones are widely underappreciated by physicians, and are often misdiagnosed as other medical or psychiatric conditions. Physicians typically fail to enquire about antibiotic use to explain with an acute presentation of new symptoms. The important adverse effects of fluoroquinolones include hypoglycemia or hyperglycemia, QTc prolongation, central nervous system toxicity, gastrointestinal, skin, musculoskeletal, cardiotoxicity, and respiratory effects, phototoxicity, tendinopathy, angioedema, and Clostridium difficile infections. A further factor that leads to misdiagnosis of quinolone adverse effects is that some symptoms can persist or occur for the first time quite some time after a course of quinolone has been finished, so inquiring about distant-past use of quinolones has been recommended. Quinolones are probably the worst offending antibiotic for causing C. difficile infections. Some of the adverse effects can present similar to acute dementia, confusion, and psychosis. Quinolones are a common cause of cerebral dysfunction, with neuropsychiatric disturbances being the most common quinolone adverse effects. One study found that, of all drug classes prescribed by doctors including psychotropic drugs, fluoroquinolones were the most common cause of neuropsychiatric adverse effects.[89]
Patent extensions
Under the George W. Bush administration (2001–2008), patent extension legislation that allowed Bayer AG, as well as other drug companies, a six-month patent extension for testing their products for safety in children was signed into law. It has been estimated that Bayer AG's revenue increased an extra $358 million due to ciprofloxacin's pediatric patent extension. The legislation was drafted after extensive lobbying of numerous members of Congress by Bayer AG and others. One of the four sponsors of this legislation was Chris Dodd (D-CT), who, at the time, ranked as one of the top three beneficiaries of campaign contributions by drug companies. Sen. Edward Kennedy (D-MA), who chaired the committee with jurisdiction over the bill, refused to fight over the language that (if it had been included) would have reduced the drug company's profits due to these patent extensions. The reasons for Sen. Edward Kennedy's decision not to fight for the inclusion of this language were not made known.[90]
The results of these pediatric trials indicated that arthropathy occurred more frequently in patients that received ciprofloxacin (within these studies). The affected joints included the knees, elbows, ankles, hips, wrists, and shoulders of the pediatric patients. In one study, at six weeks arthropathy was seen in 9.3% of ciprofloxacin patients. These rates increased significantly after one year to 13.7% of the ciprofloxacin patients. Such arthropathy occurred more frequently in patients treated with ciprofloxacin than any other control drug, regardless of whether they received IV ciprofloxacin or the oral version of the drug. Ciprofloxacin patients reported more than one event and on more than one occasion when compared to the control patients. The overall incidence of adverse events at six weeks was 41% in those patients being treated with ciprofloxacin. Serious adverse events were seen in 7.5% of these patients and 3% of the patients discontinued the drug due to adverse events. Despite these results the FDA stated that “The data support updating the package insert to include safety; and treatment recommendations for pediatric patients between 1 and 17 years of age with complicated urinary tract infection or pyelonephritis.”[91]
Within a 2005 memo, the FDA reviewed seventeen unique pediatric cases reported to the FDA during the thirteen-month period after the pediatric exclusivity for ciprofloxacin had been granted. During this period, there was one report of death, two of disability, and four of hospitalization. The disabilities involved the inability to walk (in a 12-year-old female patient) and the inability to run (in a 12-year-old male patient). The hospital admissions were for pseudomembranous colitis, pancytopenia, tendonitis, and Stevens Johnson syndrome. The female patient received 5 weeks of ciprofloxacin oral therapy at the recommended doses. Even though ciprofloxacin was discontinued, she could not stand or ambulate and required a wheelchair one month later. These seventeen unique pediatric cases showed mostly hematological, musculoskeletal, allergic/hypersensitivity, and central nervous system adverse events. It does not appear that this executive summary was ever released to the medical community.[92]
- Economic impact: adverse reactions:
The adverse drug reaction profile of ciprofloxacin and other fluoroquinolone drugs has spawned a grass-roots movement of those so affected to lobby for Black Box Warnings and Dear Doctor Letters as well as the petitioning of the FDA for the removal of some fluoroquinolone drugs from clinical practice.[49][54][93][94][95][96][97]
Current litigation
The effectiveness and the proven clinical need for the drugs found within this class have rarely been called into question. They have a proven track record with regard to eradicating bacterial infections and are to be considered an essential tool within the medical community. However, there is controversy concerning the safety profile of quinolones, as well as their proper use.
At present, there is a significant number of cases pending before the United States District Court, District of Minnesota, involving the drug Levaquin. On June 13, 2008 a Judicial Panel On Multidistrict Litigation (MDL) granted the Plaintiffs’ motion to centralize individual and class-action lawsuits involving Levaquin in the District of Minnesota over objection of Defendants, Johnson and Johnson / Ortho McNeil.[39]
Most recently, on July 6, 2009, the New Jersey Supreme Court had also designated litigation over Levaquin as a mass tort and has assigned it to an Atlantic County, N.J., judge. The suits charge that the drug has caused Achilles tendon ruptures and other permanent damage.[98]
Several class action lawsuits had been filed in regards to the adverse reactions suffered by those exposed to ciprofloxacin during the anthrax scare of 2001, as well.
Generations
Researchers divide the quinolones into generations based on their antibacterial spectrum.[99][100] The earlier-generation agents are, in general, more narrow-spectrum than the later ones, but there is no standard employed to determine which drug belongs to which generation. The only universal standard applied is the grouping of the non-fluorinated drugs found within this class (quinolones) within the first-generation heading. As such, there exists a wide variation within the literature dependent upon the methods employed by the authors. Some researchers group these drugs by patent dates, some by a specific decade (i.e., '60s, '70s, '80s, etc.), and others by the various structural changes.
The first generation is rarely used today. Nalidixic acid was added to the OEHHA Prop 65 list as a carcinogen on May 15, 1998.[101] A number of the second-, third-, and fourth-generation drugs have been removed from clinical practice due to severe toxicity issues or discontinued by their manufacturers. The drugs most frequently prescribed today consist of Avelox (moxifloxacin), Cipro (ciprofloxacin), Levaquin (levofloxacin), and, to some extent, their generic equivalents.
First-generation
- cinoxacin (Cinobac) (Removed from clinical use)[102]
- flumequine (Flubactin) (Genotoxic carcinogen)(Veterinary use)
- nalidixic acid (NegGam, Wintomylon)[102] (Genotoxic carcinogen)
- oxolinic acid (Uroxin) (Currently unavailable in the United States)
- piromidic acid (Panacid) (Currently unavailable in the United States)
- pipemidic acid (Dolcol) (Currently unavailable in the United States)
- rosoxacin (Eradacil) (Restricted use, currently unavailable in the United States)
Second-generation
The second-generation class is sometimes subdivided into "Class 1" and "Class 2".[103]
- ciprofloxacin (Ciprobay, Cipro, Ciproxin)[102][104]
- enoxacin (Enroxil, Penetrex)[102] (Removed from clinical use)
- fleroxacin (Megalone, Roquinol) (Removed from clinical use)
- lomefloxacin (Maxaquin)[102](Discontinued in the United States)
- nadifloxacin (Acuatim, Nadoxin, Nadixa) (Currently unavailable in the United States)
- norfloxacin (Lexinor, Noroxin, Quinabic, Janacin)[102](restricted use)[105]
- ofloxacin (Floxin, Oxaldin, Tarivid)[102] (Only as ophthalmic in the United States)
- pefloxacin (Peflacine) (Currently unavailable in the United States)
- rufloxacin (Uroflox) (Currently unavailable in the United States)
Third-generation
Unlike the first- and second-generations, the third-generation is active against streptococci.[103]
- balofloxacin (Baloxin) (Currently unavailable in the United States)
- gatifloxacin (Tequin) (Zymar -opth.) (Tequin removed from clinical use)[106] Sometimes reported as 4th generation.[104][107]
- grepafloxacin (Raxar) (Removed from clinical use)
- levofloxacin (Cravit, Levaquin)[102][104]
- moxifloxacin (Avelox,Vigamox)[102](restricted use).[108] Sometimes reported as 4th generation.[104][109]
- pazufloxacin (Pasil, Pazucross) (Currently unavailable in the United States)
- sparfloxacin (Zagam)[102](restricted use),[110]
- temafloxacin (Omniflox) (Removed from clinical use)[111]
- tosufloxacin (Ozex, Tosacin) (Currently unavailable in the United States)
Fourth-generation
- clinafloxacin[104](Currently unavailable in the United States)
- gemifloxacin (Factive)(Currently unavailable in the United States)[www.factive.com]
- sitafloxacin (Gracevit) (Currently unavailable in the United States)
- trovafloxacin (Trovan) (Removed from clinical use)[102][104]
- prulifloxacin (Quisnon) (Currently unavailable in the United States)
In development
- garenoxacin (Geninax)(Application withdrawn due to toxicity issues)
- delafloxacin
Veterinary use
The quinolones have been widely used in agriculture, and several agents that have veterinary but not human use exist.
- danofloxacin (Advocin, Advocid) (for veterinary use)
- difloxacin (Dicural, Vetequinon) (for veterinary use)
- enrofloxacin (Baytril) (for veterinary use)
- ibafloxacin (Ibaflin) (for veterinary use)
- marbofloxacin (Marbocyl, Zenequin) (for veterinary use)
- orbifloxacin (Orbax, Victas) (for veterinary use)
- sarafloxacin (Floxasol, Saraflox, Sarafin) (for veterinary use)
References
- ↑ Nelson JM, Chiller TM, Powers JH, Angulo FJ (April 2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story". Clin. Infect. Dis. 44 (7): 977–80. doi:10.1086/512369. PMID 17342653. http://www.journals.uchicago.edu/doi/abs/10.1086/512369?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ↑ Ivanov DV, Budanov SV (2006). "[Ciprofloxacin and antibacterial therapy of respiratory tract infections]" (in Russian). Antibiot. Khimioter. 51 (5): 29–37. PMID 17310788.
- ↑ sanofi-aventis U.S. LLC (September 2008). "NegGram® Caplets (nalidixic acid, USP)" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/014214s058lbl.pdf.
- ↑ Wentland MP: In memoriam: George Y. Lesher, Ph.D., in Hooper DC, Wolfson JS (eds): Quinolone antimicrobial agents, ed 2., Washington DC, American Society for Microbiology : XIII - XIV, 1993.
- ↑ Hooper, DC. (Mar-Apr 2001). "Emerging mechanisms of fluoroquinolone resistance." (PDF). Emerg Infect Dis 7 (2): 337–41. doi:10.3201/eid0702.010239. PMID 11294736. PMC 2631735. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2631735/pdf/11294736.pdf.
- ↑ 6.0 6.1 Muto, CA.; Jernigan, JA.; Ostrowsky, BE.; Richet, HM.; Jarvis, WR.; Boyce, JM.; Farr, BM. (May 2003). "SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus.". Infect Control Hosp Epidemiol 24 (5): 362–86. doi:10.1086/502213. PMID 12785411.
- ↑ 7.0 7.1 Dr Ralf-Peter Vonberg. "Clostridium difficile: a challenge for hospitals". European Center for Disease Prevention and Control. Institute for Medical Microbiology and Hospital Epidemiology: IHE. http://www.ihe-online.com/feature-articles/clostridium-difficile-a-challenge-for-hospitals/trackback/1/index.html. Retrieved 27 July 2009.
- ↑ Tacconelli, E.; De Angelis, G.; Cataldo, MA.; Pozzi, E.; Cauda, R. (January 2008). "Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis.". J Antimicrob Chemother 61 (1): 26–38. doi:10.1093/jac/dkm416. PMID 17986491. http://jac.oxfordjournals.org/cgi/content/full/61/1/26.
- ↑ Norris, S; Mandell, GL (1988). "The quinolones: history and overview". The quinolones: history and overview. San Diego: Academic Press Inc. pp. 1–22.
- ↑ 10.0 10.1 Stacy J. Childs, MD (2000). "Safety of the Fluoroquinolone Antibiotics: Focus on Molecular Structure". Infect Urol (USA: FQresearch) 13 (1): 3–10. http://www.fqresearch.org/causation_11.htm.
- ↑ Mittmann, N, N; Jivarj, F, F; Wong, A, A; Yoon, A, A (1 September 2002). "Oral fluoroquinolones in the treatment of pneumonia, bronchitis and sinusitis." (Free full text). The Canadian journal of infectious diseases (Journal canadien des maladies infectieuses) 13 (5): 293–300. PMID 18159405. PMC 2094884. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2094884. "There was no statistically significant difference in outcome rates between ciprofloxacin or moxifloxacin and clarithromycin".
- ↑ 12.0 12.1 Karageorgopoulos, DE.; Giannopoulou, KP.; Grammatikos, AP.; Dimopoulos, G.; Falagas, ME. (March 2008). "Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials.". CMAJ 178 (7): 845–54. doi:10.1503/cmaj.071157. PMID 18362380. PMC 2267830. http://www.cmaj.ca/cgi/content/full/178/7/845.
- ↑ Carrie, Ag; Kozyrskyj, Al (Winter 2006). "Outpatient treatment of community-acquired pneumonia: evolving trends and a focus on fluoroquinolones." (PDF). The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique 13 (1): e102–11. PMID 16585811. http://www.cjcp.ca/cjcp_05-029_e102-r101636.
- ↑ Vardakas, KZ.; Siempos, II.; Grammatikos, A.; Athanassa, Z.; Korbila, IP.; Falagas, ME. (December 2008). "Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials.". CMAJ 179 (12): 1269–77. doi:10.1503/cmaj.080358. PMID 19047608. PMC 2585120. http://www.cmaj.ca/cgi/content/full/179/12/1269.
- ↑ Le Saux, N. (March 2008). "The treatment of acute bacterial sinusitis: no change is good medicine.". CMAJ 178 (7): 865–6. doi:10.1503/cmaj.080285. PMID 18362382. PMC 2267829. http://www.cmaj.ca/cgi/content/full/178/7/865.
- ↑ Hueston, Wj, WJ (March 1997). "Antibiotics: neither cost effective nor 'cough' effective.". The Journal of family practice 44 (3): 261–5. ISSN 0094-3509. PMID 9071245.
- ↑ Liu, H.; Mulholland, SG. (July 2005). "Appropriate antibiotic treatment of genitourinary infections in hospitalized patients.". Am J Med 118 Suppl 7A: 14S–20S. doi:10.1016/j.amjmed.2005.05.009. PMID 15993673.
- ↑ Wein, Alan J.; Kavoussi, Louis R.; Novick, Andrew C.; Partin, Alan W.; Peters, Craig A. (19 March 2007). Campbell-Walsh Urology Review Manual (Ninth ed.). Saunders. ISBN 978-1416031550. http://books.google.com/?id=KGVqAAAAMAAJ.
- ↑ 19.0 19.1 MacDougall C, Guglielmo BJ, Maselli J, Gonzales R (March 2005). "Antimicrobial drug prescribing for pneumonia in ambulatory care". Emerging Infect. Dis. 11 (3): 380–4. PMID 15757551. http://www.cdc.gov/ncidod/EID/vol11no03/04-0819.htm.
- ↑ 20.0 20.1 De Sarro A, De Sarro G (March 2001). "Adverse reactions to fluoroquinolones. an overview on mechanistic aspects" (PDF). Curr. Med. Chem. 8 (4): 371–84. PMID 11172695. http://www.fqresearch.org/pdf_files/cmc.pdf.
- ↑ Owens RC, Ambrose PG (July 2005). "Antimicrobial safety: focus on fluoroquinolones". Clin. Infect. Dis. 41 Suppl 2: S144–57. doi:10.1086/428055. PMID 15942881.
- ↑ 22.0 22.1 22.2 Owens RC, Ambrose PG (July 2005). "Antimicrobial safety: focus on fluoroquinolones". Clin. Infect. Dis. 41 Suppl 2: S144–57. doi:10.1086/428055. PMID 15942881. http://www.journals.uchicago.edu/cgi-bin/resolve?CID34940.
- ↑ 23.0 23.1 Iannini PB (June 2007). "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations". Curr Med Res Opin 23 (6): 1403–13. doi:10.1185/030079907X188099. PMID 17559736.
- ↑ IRISH MEDICINES BOARD (January 2002). "DRUG SAFETY NEWSLETTER 14th Edition" (PDF). Ireland: imb.ie. http://www.imb.ie/images/uploaded/documents/Issue14.pdf. Retrieved 26 February 2009.
- ↑ Rouveix, B. (Nov-Dec 2006). "[Clinically significant toxicity and tolerance of the main antibiotics used in lower respiratory tract infections]". Med Mal Infect 36 (11-12): 697–705. doi:10.1016/j.medmal.2006.05.012. PMID 16876974.
- ↑ Falagas ME, Rafailidis PI, Rosmarakis ES (April 2007). "Arrhythmias associated with fluoroquinolone therapy". Int. J. Antimicrob. Agents 29 (4): 374–9. doi:10.1016/j.ijantimicag.2006.11.011. PMID 17241772.
- ↑ 27.0 27.1 Mehlhorn AJ, Brown DA (November 2007). "Safety concerns with fluoroquinolones". Ann Pharmacother 41 (11): 1859–66. doi:10.1345/aph.1K347. PMID 17911203.
- ↑ Lewis RJ, Mohr JF (2008). "Dysglycaemias and fluoroquinolones". Drug Saf 31 (4): 283–92. doi:10.2165/00002018-200831040-00002. PMID 18366239.
- ↑ Rubinstein E (2001). "History of quinolones and their side effects". Chemotherapy 47 Suppl 3: 3–8; discussion 44–8. doi:10.1159/000057838. PMID 11549783. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=che7c003.
- ↑ Khaliq Y, Zhanel GG (October 2005). "Musculoskeletal injury associated with fluoroquinolone antibiotics". Clin Plast Surg 32 (4): 495–502, vi. doi:10.1016/j.cps.2005.05.004. PMID 16139623.
- ↑ 31.0 31.1 Nelson, Lewis H.; Flomenbaum, Neal; Goldfrank, Lewis R.; Hoffman, Robert Louis; Howland, Mary Deems; Neal A. Lewin (2006). Goldfrank's toxicologic emergencies. New York: McGraw-Hill, Medical Pub. Division. ISBN 0-07-143763-0. http://books.google.com/?id=cvJuLqBxGUcC&pg=PA849&dq=goldfranks+Fluoroquinolone+toxicity.
- ↑ Jones SF, Smith RH (March 1997). "Quinolones may induce hepatitis" (PDF). BMJ 314 (7084): 869. PMID 9093098. PMC 2126221. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=2126221&blobtype=pdf.
- ↑ Saint F, Gueguen G, Biserte J, Fontaine C, Mazeman E (September 2000). "[Rupture of the patellar ligament one month after treatment with fluoroquinolone"] (in French). Rev Chir Orthop Reparatrice Appar Mot 86 (5): 495–7. PMID 10970974. http://www.masson.fr/masson/MDOI-RCO-09-2000-86-5-0035-1040-101019-ART7.
- ↑ Kuijper EJ, van Dissel JT, Wilcox MH, EJ (August 2007). "Clostridium difficile: changing epidemiology and new treatment options". Curr. Opin. Infect. Dis. 20 (4): 376–83. doi:10.1097/QCO.0b013e32818be71d. ISSN 0951-7375. PMID 17609596.
- ↑ Blossom DB, McDonald LC, DB (July 2007). "The challenges posed by reemerging Clostridium difficile infection". Clin. Infect. Dis. 45 (2): 222–7. doi:10.1086/518874. ISSN 1058-4838. PMID 17578783. http://www.journals.uchicago.edu/doi/full/10.1086/518874.
- ↑ Pépin J, Saheb N, Coulombe MA, et al., J (November 2005). "Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec". Clin. Infect. Dis. 41 (9): 1254–60. doi:10.1086/496986. ISSN 1058-4838. PMID 16206099. http://www.journals.uchicago.edu/doi/abs/10.1086/496986.
- ↑ Galatti L, Giustini SE, Sessa A, et al. (March 2005). "Neuropsychiatric reactions to drugs: an analysis of spontaneous reports from general practitioners in Italy". Pharmacol. Res. 51 (3): 211–6. doi:10.1016/j.phrs.2004.08.003. PMID 15661570. http://linkinghub.elsevier.com/retrieve/pii/S1043-6618(04)00213-0.
- ↑ "Public Citizen Warns of Cipro Dangers". USA: Consumer affairs. 30 August 2006. http://www.consumeraffairs.com/news04/2006/08/pubcit_cipro.html. Retrieved 7 September 2009.
- ↑ 39.0 39.1 Judge John R. Tunheim. "Levaquin MDL". USA: US Courts. http://www.mnd.uscourts.gov/MDL-Levaquin/index.shtml. Retrieved 7 September 2009.
- ↑ "FDA orders 'black box' label on some antibiotics". CNN. 2008-07-08. http://www.cnn.com/2008/HEALTH/07/08/antibiotics.risk/index.html. Retrieved 2008-07-08.
- ↑ Nardiello, S.; Pizzella, T.; Ariviello, R. (March 2002). "[Risks of antibacterial agents in pregnancy]". Infez Med 10 (1): 8–15. PMID 12700435.
- ↑ Noel, GJ.; Bradley, JS.; Kauffman, RE.; Duffy, CM.; Gerbino, PG.; Arguedas, A.; Bagchi, P.; Balis, DA. et al. (October 2007). "Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders.". Pediatr Infect Dis J 26 (10): 879–91. doi:10.1097/INF.0b013e3180cbd382. PMID 17901792.
- ↑ Leibovitz, E.; Dror, Yigal (February 2006). "The use of fluoroquinolones in children.". Curr Opin Pediatr 18 (1): 64–70. doi:10.1097/01.mop.0000192520.48411.fa. PMID 16470165.
- ↑ Janssen Pharmaceutica (September 2008). "HIGHLIGHTS OF PRESCRIBING INFORMATION" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf.
- ↑ Scherer, K.; Bircher, AJ. (January 2005). "Hypersensitivity reactions to fluoroquinolones.". Curr Allergy Asthma Rep 5 (1): 15–21. doi:10.1007/s11882-005-0049-1. PMID 15659258.
- ↑ Bailey RR, Natale R, Linton AL, RR (October 1972). "Nalidixic acid arthralgia". Can Med Assoc J 107 (7): 604 passim. ISSN 0008-4409. PMID 4541768.
- ↑ Bailey RR, Kirk JA, Peddie BA, RR (July 1983). "Norfloxacin-induced rheumatic disease" (Free full text). N Z Med J 96 (736): 590. ISSN 0028-8446. PMID 6223241. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+389-08-2.
- ↑ Szarfman A, Chen M, Blum MD, A (January 1995). "More on fluoroquinolone antibiotics and tendon rupture" (letter). N Engl J Med 332 (3): 193. doi:10.1056/NEJM199501193320319. ISSN 0028-4793. PMID 7800023.
- ↑ 49.0 49.1 "Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399)". Public Citizen. August 1, 1996. http://www.citizen.org/publications/release.cfm?ID=6595. Retrieved 27 December 2008.
- ↑ "Reports of adverse events with fluoroquinolones". FDA Medical Bulletin 26 (3). October 1996. http://www.fqresearch.org/text_documents/FDA_Medical_Bulletin_1996.doc. Retrieved 27 December 2008.
- ↑ 51.0 51.1 Office of the Illinois Attorney General (August 29, 2006). "Madigan, Public Citizen, petition FDA for "Black Box" warning regarding potential adverse effects of certain popular antibiotics". Press release. http://www.illinoisattorneygeneral.gov/pressroom/2006_08/20060829.html. Retrieved 27 December 2008.
- ↑ Lisa Madigan; OFFICE OF THE ATTORNEY GENERAL (18 May 2005). "CITIZEN PETITION" (PDF). USA: State Of Illinois. http://fqresearch.org/pdf_files/fda_response.pdf. Retrieved 27 December 2008.
- ↑ Jane A. Axelrad; FDA (16 November 2005). "Re: Docket No. 2005P-0205" (PDF). USA: FQresearch. http://fqresearch.org/pdf_files/illinois.pdf. Retrieved 27 December 2008.
- ↑ 54.0 54.1 "Public Citizen Petitions the FDA to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781)". Public Citizen. August 29, 2006. http://www.citizen.org/publications/release.cfm?ID=7453. Retrieved 2008-12-27.
- ↑ "Public Citizen v. Food and Drug Administration (FDA) (Fluoroquinolone)". Public Citizen. January 3, 2008. http://www.citizen.org/litigation/forms/cases/CaseDetails.cfm?cID=444. Retrieved 2008-12-27.
- ↑ Ravn, Karen (August 18, 2008). "Behind the FDA’s ‘black box’ warnings". Los Angeles Times. http://articles.latimes.com/2008/aug/18/health/he-closer18. Retrieved 2008-12-27.
- ↑ U.S. Food and Drug Administration (2008-07-08). "FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs". Press release. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01858.html. Retrieved 2008-10-11.
- ↑ "Drugs@FDA". USA: FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Retrieved 12 August 2009.
- ↑ MacCarthy, Paul (October 22, 2008). "Important Change in the Avelox (moxifloxacin hydrochloride) and Cipro (ciprofloxacin) Complete Prescribing Information – Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture". Bayer HealthCare Pharmaceuticals. http://www.cipro.com/html/pdf/dhpl.pdf. Retrieved 2008-12-27.
- ↑ Rosenthal, Norman (November 2008). "Important Change in the LEVAQUIN (Ievofloxacin) Complete Prescribing Information -Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture". Ortho-McNeil Janssen Scientific Affairs, LLC. http://www.fqresearch.org/pdf_files/Levaquin_11_2008_ortho_mcneil_dear_dr_letter.pdf. Retrieved 2008-12-27.
- ↑ Schaumann, R.; Rodloff, A. C. (January 2007). "Activities of Quinolones Against Obligately Anaerobic Bacteria" (PDF). Anti-Infective Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry - Anti-Infective Agents) (Bentham Science Publishers) 6 (1): 49–56. http://www.bentham.org/cmcaia/sample/cmcaia%206-1/0004Y.pdf.
- ↑ Chang Y.H.; Se H.K.; Young K.K (22 July 1997). "Novel 5-amino-6-methylquinolone antibacterials: A new class of non-6-fluoroquinolones". Bioorganic & Medicinal Chemistry Letters (Elsevier) 7 (14): 1875–1878. doi:10.1016/S0960-894X(97)00324-7.
- ↑ CANN HM, VERHULST HL (January 1961). "Fatal acute chloroquine poisoning in children". Pediatrics 27 (1): 95–102. PMID 13690445. http://pediatrics.aappublications.org/cgi/content/abstract/27/1/95.
- ↑ Murray, Robert K.; Granner, Darryl K.; Mayes, Peter A.; Rodwell, Victor W. (1 July 2006). "Protein Synthesis and the Genetic Code". Harper's Illustrated Biochemistry (27 ed.). McGraw-Hill Medical. p. 378. ISBN 0071461973. http://books.google.com/?id=QrJCZ1v5T6AC. "The most useful members of this class of antibiotics (eg tetracyclines, lincomycin, erythromycin and chloramphenicol) do not interact with components of eukaryotic ribosomal particles and thus are not toxic to eukaryotes..."
- ↑ Elsea, SH.; Osheroff, N.; Nitiss, JL. (July 1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast." (pdf). J Biol Chem 267 (19): 13150–3. PMID 1320012. http://www.jbc.org/content/267/19/13150.full.pdf.
- ↑ Bergan T.; Bayer (1988). "Pharmacokinetics of fluorinated quinolones". Academic Press: 119–154.
- ↑ Bergan T; Dalhoff A, Thorsteinsson SB (1985). A review of the pharmacokinetics and tissue penetration of ciprofloxacin. pp. 23–36.
- ↑ Castora, FJ.; Vissering, FF.; Simpson, MV. (September 1983). "The effect of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria.". Biochim Biophys Acta 740 (4): 417–27. PMID 6309236.
- ↑ Kaplowitz, Neil (2005). "Hepatology highlights". Hepatology 41: 227. doi:10.1002/hep.20596.
- ↑ Enzmann, H.; Wiemann, C.; Ahr, HJ.; Schlüter, G. (April 1999). "Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver.". Mutat Res 425 (2): 213–24. PMID 10216214.
- ↑ MCQUEEN CA.; WILLIAMS GM. (1987). "Effects of quinolone antibiotics in tests for genotoxicity". Am. J. Med 82 (Suppl. 4A): 94–96.
- ↑ Holden, HE.; Barett, JF.; Huntington, CM.; Muehlbauer, PA.; Wahrenburg, MG. (1989). "Genetic profile of a nalidixic acid analog: a model for the mechanism of sister chromatid exchange induction.". Environ Mol Mutagen 13 (3): 238–52. doi:10.1002/em.2850130308. PMID 2539998.
- ↑ Suto, MJ.; Domagala, JM.; Roland, GE.; Mailloux, GB.; Cohen, MA. (December 1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity.". J Med Chem 35 (25): 4745–50. doi:10.1021/jm00103a013. PMID 1469702.
- ↑ "Moderate Interaction: Quinolones/Corticosteroids". Medscape. http://www.medscape.com/druginfo/monoinfobyid?cid=med&monotype=druginter&monoid=1443&mononame=QUINOLONES%2FCORTICOSTEROIDS&drugid=17879&drugname=Avelox+Oral&intertype=mod. Retrieved September 2, 2008.
- ↑ Cohen JS (December 2001). "Peripheral Neuropathy Associated with Fluoroquinolones" (PDF). Ann Pharmacother 35 (12): 1540–7. doi:10.1345/aph.1Z429. PMID 11793615. http://fqvictims.org/fqvictims/News/neuropathy/Neuropathy.pdf.
- ↑ 76.0 76.1 "Fluoroquinolone Adverse Effects and Drug Interactions". Medscape. http://www.medscape.com/viewarticle/418295_4. Retrieved September 2, 2008.
- ↑ Fluoroquinolone Adverse Effects and Drug Interactions: Drug-Drug Interactions Author from: the Department of Pharmacy Practice, School of Pharmacy, University of Colorado Health Sciences Center, Denver, Colorado. Retrieved on Dec 17, 2009
- ↑ 78.0 78.1 Swedish environmental classification of pharmaceuticals Facts for prescribers (Fakta för förskrivare)
- ↑ M Jacobs, Worldwide Overview of Antimicrobial Resistance. International Symposium on Antimicrobial Agents and Resistance 2005.
- ↑ Nelson, JM.; Chiller, TM.; Powers, JH.; Angulo, FJ. (April 2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story." (PDF). Clin Infect Dis 44 (7): 977–80. doi:10.1086/512369. PMID 17342653. http://www.journals.uchicago.edu/doi/pdf/10.1086/512369.
- ↑ Jim Hoover, for Bayer Corporation, Alaska Pharmacy and Therapeutics Committee March 19, 2004
- ↑ Froom J, Culpepper L, Jacobs M, et al. (July 1997). "Antimicrobials for acute otitis media? A review from the International Primary Care Network" (PDF). BMJ 315 (7100): 98–102. PMID 9240050. PMC 2127061. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=2127061&blobtype=pdf.
- ↑ 83.0 83.1 Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS (March 2005). "Fluoroquinolone prescribing in the United States: 1995 to 2002". The American Journal of Medicine 118 (3): 259–68. doi:10.1016/j.amjmed.2004.09.015. PMID 15745724.
- ↑ K08 HS14563 and HS11313
- ↑ Neuhauser, MM; Weinstein, RA; Rydman, R; Danziger, LH; Karam, G; Quinn, JP (2003). "Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use.". JAMA : the journal of the American Medical Association 289 (7): 885–8. doi:10.1001/jama.289.7.885. PMID 12588273. http://www.ahrq.gov/research/nov07/1107RA29.htm. "From 1995 to 2002, inappropriate antibiotic prescribing for acute respiratory infections, which are usually caused by viruses and thus are not responsive to antibiotics, declined from 61 to 49 percent. However, the use of broad-spectrum antibiotics such as the fluoroquinolones, jumped from 41 to 77 percent from 1995 to 2001. Overuse of these antibiotics will eventually render them useless for treating antibiotic-resistant infections, for which broad-spectrum antibiotics are supposed to be reserved.".
- ↑ Lautenbach E, Larosa LA, Kasbekar N, Peng HP, Maniglia RJ, Fishman NO (March 2003). "Fluoroquinolone utilization in the emergency departments of academic medical centers: prevalence of, and risk factors for, inappropriate use". Arch. Intern. Med. 163 (5): 601–5. doi:10.1001/archinte.163.5.601. PMID 12622607. http://archinte.ama-assn.org/cgi/content/full/163/5/601.
- ↑ Robicsek A, Jacoby GA, Hooper DC (October 2006). "The worldwide emergence of plasmid-mediated quinolone resistance". Lancet Infect Dis 6 (10): 629–40. doi:10.1016/S1473-3099(06)70599-0. PMID 17008172. http://linkinghub.elsevier.com/retrieve/pii/S1473-3099(06)70599-0.
- ↑ Beringer PM, Wong-Beringer A, Rho JP (January 1998). "Economic aspects of antibacterial adverse effects". Pharmacoeconomics 13 (1 Pt 1): 35–49. doi:10.2165/00019053-199813010-00004. PMID 10175984. "Indirect costs as a result of reduced quality of life or loss of productivity are certainly not reflected in the acquisition costs of antimicrobials.".
- ↑ James R. Roberts (October 2008). "Adverse Reactions to Fluoroquinolones". Emergency Medicine News (Emergency Medicine News) 30 (10): 16–18. doi:10.1097/01.EEM.0000338244.41795.9c (inactive 2009-11-16). http://journals.lww.com/em-news/Fulltext/2008/10000/Adverse_Reactions_to_Fluoroquinolones_.23.aspx.
- ↑ Public Citizen. "Patently Offensive: Congress Set to Extend Monopoly Patents for Cipro and Other Drugs". USA: citizen.org. http://www.citizen.org/print_article.cfm?ID=6435. Retrieved 13 August 2009.
- ↑ Meyer, Joette (16 March 2004). "Division of Special Pathogen and Immunologic Drug Products - Summary of Clinical Review of Studies Submitted in Response to a Pediatric Written Request" (PDF). USA: FDA. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4152b1_03_03_Cipro%20medical.pdf. Retrieved 31 August 2009.
- ↑ Farinas, Evelyn R; DEPARTMENT OF HEALTH AND HUMAN (1 March 2005). "Consult: One-Year Post Pediatric Exclusivity Postmarketing Adverse Events Review" (PDF). USA: FDA. http://www.fda.gov/OHRMS/DOCKETS/AC/05/briefing/2005-4152b1_03_01_Cipro%20AE.pdf. Retrieved 31 August 2009.
- ↑ Public Citizen; Michael T. Kickpatrick (3 January 2008). "In The United States District Court For The District Of Columbia" (PDF). USA: Carey & Danis, LLC. Archived from the original on 2008. http://www.fqresearch.org/fda_suit.htm.
- ↑ Lisa Madigan (18 May 2005). "Office Of The Attorney General State Of Illinois, CITIZEN PETITION" (PDF). USA: FDA. Archived from the original on 2005. http://www.fqresearch.org/pdf_files/fda_response.pdf.
- ↑ Joseph Baker; Sidney Wolfe, Peter Lurie (1 May 2006). "Petition to the FDA to Immediately Ban the Antibiotic Gatifloxacin (Tequin) (HRG Publication #1768)". USA: Public Citizen. http://www.citizen.org/publications/release.cfm?ID=7430.
- ↑ "Letter to the Food and Drug Administration to immediately ban the antibiotic trovafloxacin (Trovan) (HRG Publication #1485)". USA: Public Citizen. 3 June 1999. http://www.citizen.org/publications/release.cfm?ID=6684.
- ↑ Larry D. Sasich,; Sidney M. Wolfe, Allison Zieve, Patricia Christen, Ben Christen (9 June 1998). "Petition to the Food and Drug Administration to immediately stop the distribution of dangerous, misleading prescription drug information to the public. (HRG Publication #1442)". USA: Public Citizen. http://www.citizen.org/publications/release.cfm?ID=6639.
- ↑ Charles Toutant (6 July 2009). "Litigation Over Johnson & Johnson Antibiotic Levaquin Designated N.J. Mass Tort". New Jersey Law Journal. http://www.law.com/jsp/article.jsp?id=1202431984309.
- ↑ Ball P (2000). "Quinolone generations: natural history or natural selection?". J. Antimicrob. Chemother. 46 Suppl T1 (Supplement 3): 17–24. PMID 10997595. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=10997595.
- ↑ "New Classification and Update on the Quinolone Antibiotics - May 1, 2000 - American Academy of Family Physicians". http://www.aafp.org/afp/20000501/2741.html. Retrieved 2008-03-18.
- ↑ [Nalidixic Acid, case number 389-08-02, listing mechanism AB, NTP (1989b]
- ↑ 102.00 102.01 102.02 102.03 102.04 102.05 102.06 102.07 102.08 102.09 102.10 "Quinolones: A Comprehensive Review - February 1, 2002 - American Family Physician". http://www.aafp.org/afp/20020201/455.html.
- ↑ 103.0 103.1 Oliphant CM, Green GM (February 2002). "Quinolones: a comprehensive review". Am Fam Physician 65 (3): 455–64. PMID 11858629. http://www.aafp.org/afp/20020201/455.html.
- ↑ 104.0 104.1 104.2 104.3 104.4 104.5 Paul G. Ambrose; Robert C. Owens, Jr (1 March 2000). "Clinical Usefulness Of Quinolones". Seminars in Respiratory and Critical Care Medicine (Medscape). http://www.medscape.com/viewarticle/410872_6.
- ↑ The European Medicines Agency (24 July 2008). "EMEA Restricts Use of Oral Norfloxacin Drugs in UTIs". Doctor's Guide. http://www.docguide.com/news/content.nsf/news/852571020057CCF68525749000687709.
- ↑ Schmid, Randolph E. (May 1, 2006). "Drug Company Taking Tequin Off Market". Associated Press. http://www.sfgate.com/cgi-bin/article.cgi?file=/news/archive/2006/05/01/national/w120748D88.DTL&type=health. Retrieved 2006-05-01.
- ↑ Fiscella RG, Lewis CC, Jensen MK (October 2007). "Topical ophthalmic fourth-generation fluoroquinolones: Appropriate use and cost considerations". Am J Health Syst Pharm 64 (19): 2069–73. doi:10.2146/ajhp060667. PMID 17893419. http://www.ajhp.org/cgi/pmidlookup?view=long&pmid=17893419.
- ↑ The European Medicines Agency (EMEA); Danish Medicines Agency (24 July 2008). "EMEA recommends restricting the use of oral moxifloxacin-containing medicines". http://www.dkma.dk/1024/visUKLSArtikel.asp?artikelID=13835.
- ↑ Miravitlles M, Anzueto A (July 2008). "Moxifloxacin: a respiratory fluoroquinolone". Expert Opin Pharmacother 9 (10): 1755–72. doi:10.1517/14656566.9.10.1755. PMID 18570608.
- ↑ UN (2005). "Consolidated list of products - Pharmaceuticals 12th issue" (PDF). United Nations. http://www.un.org/esa/coordination/CL12.pdf.
- ↑ Paul G. Ambrose; Robert C. Owens, Jr (1 March 2000). "New Antibiotics in Pulmonary and Critical Care Medicine: Classification Of Quinolones By Generation". USA: Medscape. http://www.medscape.com/viewarticle/410872_4.
External links
- Quinolone at the Open Directory Project
- Fact Sheet: Quinolones
- Information to healthcare professionals on fluoroquinolone safety from the U.S. Food and Drug Administration
- Fluoroquinolones "Family Practice Notebook" entry page for Fluoroquinolones
- Structure Activity Relationships "Antibacterial Agents; Structure Activity Relationships," André Bryskier MD
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||