Plutonium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
silvery white |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | plutonium, Pu, 94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /pluːˈtoʊniəm/ ploo-TOE-nee-əm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | actinide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | n/a, 7, f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | (244)g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f6 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 24, 8, 2 (Image) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 19.816 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 16.63 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 912.5 K, 639.4 °C, 1182.9 °F | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3505 K, 3228 °C, 5842 °F | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.82 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 333.5 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 35.5 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 7, 6, 5, 4, 3 (amphoteric oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.28 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 584.7 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 159 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 187±1 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | monoclinic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 1.460 µΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 6.74 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 46.7 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2260 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 96 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 43 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-07-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of plutonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Plutonium (pronounced /pluːˈtoʊniəm/ ploo-TOE-nee-əm) is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-white appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen and silicon. When exposed to moist air, it forms oxides and hydrides that expand the sample up to 70% in volume, which in turn flake off as a powder that can spontaneously ignite. It is also a radioactive poison that accumulates in bone marrow. These and other properties make the handling of plutonium dangerous.
Plutonium is the heaviest naturally occurring or primordial element. The most stable isotope of plutonium is plutonium-244, with a half-life of about 80 million years, long enough to be found in trace quantities in nature. [3]
The most important isotope of plutonium is plutonium-239, with a half-life of 24,100 years. Plutonium-239 is the isotope most useful for nuclear weapons. Plutonium-239 and 241 are fissile, meaning the nuclei of their atoms can break apart by being bombarded by slow moving thermal neutrons, releasing energy, gamma radiation and more neutrons. These can therefore sustain a nuclear chain reaction, leading to applications in nuclear weapons and nuclear reactors.
Plutonium-238 has a half-life of 88 years and emits alpha particles. It is a heat source in radioisotope thermoelectric generators, which are used to power some spacecraft.
Plutonium-240 has a high rate of spontaneous fission, raising the neutron flux of any sample it is in. The presence of plutonium-240 limits a sample's usability for weapons or reactor fuel, and determines its grade.
Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized reactors.
Plutonium was first synthesized in 1940 by a team led by Glenn T. Seaborg and Edwin McMillan at the University of California, Berkeley laboratory by bombarding uranium-238 with deuterons. McMillan named the new element after Pluto, and Seaborg suggested the symbol Pu as a joke. Trace amounts of plutonium were subsequently discovered in nature. Producing plutonium in quantity for the first time was a major part of the Manhattan Project during World War II, which developed the first atomic bombs. The first nuclear test, "Trinity" (July 1945), and the second atomic bomb used to destroy a city (Nagasaki, Japan, in August 1945), "Fat Man", both had cores of plutonium-239. Human radiation experiments studying plutonium were conducted without informed consent, and a number of criticality accidents, some lethal, occurred during and after the war. Disposal of plutonium waste from nuclear power plants and dismantled nuclear weapons built during the Cold War is a nuclear-proliferation and environmental concern. Other sources of plutonium in the environment are fallout from numerous above-ground nuclear tests (now banned).
Contents |
Characteristics
Physical properties
Plutonium, like most metals, has a bright silvery appearance at first, much like nickel, but it oxidizes very quickly to a dull gray, although yellow and olive green are also reported.[4][5] At room temperature plutonium is in its α form (alpha). This, the most common structural form of the element (allotrope), is about as hard and brittle as grey cast iron unless it is alloyed with other metals to make it soft and ductile. Unlike most metals, it is not a good conductor of heat or electricity. It has a low melting point (640 °C) and an unusually high boiling point (3,327 °C).[4]
Alpha particle emission, which is the release of high-energy helium nuclei, is the most common form of radiation given off by plutonium.[6] A typical nuclear weapon core of 5 kg contains about 12.5 × 1024 atoms. With a half life of 24,100 years, about 11.5 × 1012 of its atoms decay each second by emitting a 5.157 MeV alpha particle. This amounts to 9.68 watts of energy. Heat produced by the deceleration of these alpha particles make it warm to the touch.[7][8]
Resistivity is a measure of how strongly a material opposes the flow of electric current. The resistivity of plutonium at room temperature is very high for a metal, and it gets even higher with lower temperatures, which is unusual for metals.[9] This trend continues down to 100 K, below which resistivity rapidly decreases for fresh samples.[9] Resistivity then begins to increase with time at around 20 K due to radiation damage, with the rate dictated by the isotopic composition of the sample.[9]
Because of self-irradiation, a sample of plutonium fatigues throughout its crystal structure, meaning the ordered arrangement of its atoms becomes disrupted by radiation with time.[10] However, self-irradiation can also lead to annealing which counteracts some of the fatigue effects as temperature increases above 100 K.[11]
Unlike most materials, plutonium increases in density when it melts, by 2.5%, but the liquid metal exhibits a linear decrease in density with temperature.[9] Near the melting point, the liquid plutonium has also very high viscosity and surface tension as compared to other metals.[10]
Allotropes
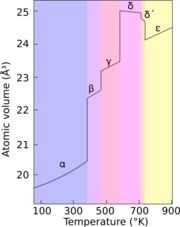
Plutonium normally has six allotropes and forms a seventh (zeta, ζ) at high temperature within a limited pressure range.[12] These allotropes, which are different structural modifications or forms of an element, have very similar internal energies but significantly varying densities and crystal structures. This makes plutonium very sensitive to changes in temperature, pressure, or chemistry, and allows for dramatic volume changes following phase transitions from one allotropic form to another.[10] Densities of the different allotropes vary from 16.00 g/cm3 to 19.86 g/cm3.[13]
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. For example, the α form exists at room temperature in unalloyed plutonium. It has machining characteristics similar to cast iron but changes to the plastic and malleable β form (beta) at slightly higher temperatures.[14] The reasons for the complicated phase diagram are not entirely understood. The α form has a low-symmetry monoclinic structure, hence its brittleness, strength, compressibility, and poor conductivity.[12]
Plutonium in the δ form normally exists in the 310 °C to 452 °C range but is stable at room temperature when alloyed with a small percentage of gallium, aluminium, or cerium, enhancing workability and allowing it to be welded.[14] The delta form has more typical metallic character, and is roughly as strong and malleable as aluminium.[12] In fission weapons, the explosive shock waves used to compress a plutonium core will also cause a transition from the usual delta phase plutonium to the denser alpha form, significantly helping to achieve supercriticality.[15] The ε phase, the highest temperature solid allotrope, exhibits anomalously high atomic self-diffusion compared to other elements.[10]
Nuclear fission

Plutonium is an element where the 5f electrons are the transition border between delocalized and localized; it is therefore considered one of the most complex elements.[16] It is a radioactive actinide metal whose isotope, plutonium-239, is one of the three primary fissile isotopes[17] (uranium-233 and uranium-235 are the other two);[18] plutonium-241 is also highly fissile. To be considered fissile, an isotope's atomic nucleus must be able to break apart or fission when struck by a slow moving neutron, and to release enough additional neutrons in the process to sustain the nuclear chain reaction by splitting further nuclei.
Plutonium-239 has a multiplication factor (k) larger than one, which means that if the metal is present in sufficient mass and with an appropriate geometry (e.g., a compressed sphere), it can form a critical mass.[19] During fission, a fraction of the binding energy, which holds a nucleus together, is released as a large amount of thermal, electromagnetic and kinetic energy; a kilogram of plutonium-239 can produce an explosion equivalent to 20,000 tons of TNT.[7] It is this energy that makes plutonium-239 useful in nuclear weapons and reactors.
The presence of the isotope plutonium-240 in a sample limits its nuclear bomb potential, as plutonium-240 has a relatively high spontaneous fission rate (~440 fissions per second per gram—over 1,000 neutrons per second per gram[20]), raising the background neutron levels and thus increasing the risk of predetonation.[21] Plutonium is identified as either weapons-grade, fuel grade, or power reactor grade based on the percentage of plutonium-240 that it contains. Weapons-grade plutonium contains less than 7% plutonium-240. Fuel grade plutonium contains from 7% to less than 19%, and power reactor grade contains 19% or more plutonium-240. Supergrade plutonium, with less than 4% of plutonium-240, is used in U.S. Navy weapons stored in proximity to ship and submarine crews, due to its lower radioactivity.[22] The isotope plutonium-238 is not fissile but can undergo nuclear fission easily with fast neutrons as well as alpha decay.[7]
Isotopes and synthesis
Twenty radioactive isotopes of plutonium have been characterized. The longest-lived are plutonium-244, with a half-life of 80.8 million years, plutonium-242, with a half-life of 373,300 years, and plutonium-239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight metastable states, though none are stable and all have half-lives less than one second.[6]
The isotopes of plutonium range in mass number from 228 to 247. The primary decay modes of isotopes with mass numbers lower than the most stable isotope, plutonium-244, are spontaneous fission and α emission, mostly forming uranium (92 protons) and neptunium (93 protons) isotopes as decay products (neglecting the wide range of daughter nuclei created by fission processes). The primary decay mode for isotopes with mass numbers higher than plutonium-244 is β emission, mostly forming americium (95 protons) isotopes as decay products. Plutonium-241 is the parent isotope of the neptunium decay series, decaying to americium-241 via β or electron emission.[6][7]
Plutonium-238 and 239 are the most-widely synthesized isotopes.[7] Plutonium-239 is synthesized via the following reaction using uranium (U) and neutrons (n) via beta decay (β−) with neptunium (Np) as an intermediate:[23]
Neutrons from the fission of uranium-235 are captured by uranium-238 nuclei to form uranium-239; a beta decay converts a neutron into a proton to form Np-239 (half-life 2.36 days) and another beta decay forms plutonium-239.[24] Workers on the Tube Alloys project had predicted this reaction theoretically in 1940.
Plutonium-238 is synthesized by bombarding uranium-238 with deuterons (D, the nuclei of heavy hydrogen) in the following reaction:[25]
In this process, a deuteron hitting uranium-238 produces two neutrons and neptunium-238, which spontaneously decays by emitting negative beta particles to form plutonium-238.
Decay heat and fission properties
Plutonium isotopes undergo radioactive decay, which produces decay heat. Different isotopes produce different amounts of heat per mass. The decay heat is usually listead as watt/kilogram, or milliwatt/gram. In case of larger pieces of plutonium (e.g. a weapon pit) and inadequate heat removal the resulting self-heating may be significant. All isotopes produce weak gamma on decay.
| Isotope | Decay mode | Half-life (years) | Decay heat (W/kg) | Spontaneous fission neutrons (1/(g·s)) | Comment |
|---|---|---|---|---|---|
| Pu-238 | alpha to U-234 | 87.74 | 560 | 2600 | Very high decay heat. Even in small amounts can cause significant self-heating. Used on its own in radioisotope thermoelectric generators. |
| Pu-239 | alpha to U-235 | 24100 | 1.9 | 0.022 | The principal fissile isotope in use. |
| Pu-240 | alpha to U-236, spontaneous fission | 6560 | 6.8 | 910 | The principal impurity of the Pu-239 isotope. The plutonium grade is usually listed as percentage of Pu-240. High spontaneous fission hinders use in nuclear weapons. |
| Pu-241 | beta, to Am-241 | 14.4 | 4.2 | 0.049 | Decays to americium-241; its buildup presents a radiation hazard in older samples. |
| Pu-242 | alpha to U-238 | 376000 | 0.1 | 1700 |
Americium-241, the decay product of plutonium-241, has half-life of 430 years, 1.2 spontaneous fissions per gram per second, and decay heat of 114 watts per kilogram. As its decay produces highly penetrative gamma rays, its presence in plutonium, determined by the original concentration of plutonium-241 and the sample age, increases the radiation exposure of surrounding structures and personnel.
Compounds and chemistry

At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized.[7] The element displays four common ionic oxidation states in aqueous solution and one rare one:[13]
- Pu(III), as Pu3+ (blue lavender)
- Pu(IV), as Pu4+ (yellow brown)
- Pu(V), as PuO2+ (pink?)[note 1]
- Pu(VI), as PuO22+ (pink orange)
- Pu(VII), as PuO53− (green)–the heptavalent ion is rare
The color shown by plutonium solutions depends on both the oxidation state and the nature of the acid anion.[27] It is the acid anion that influences the degree of complexing—how atoms connect to a central atom—of the plutonium species.
Metallic plutonium is produced by reacting plutonium tetrafluoride with barium, calcium or lithium at 1200 °C.[28] It is attacked by acids, oxygen, and steam but not by alkalis and dissolves easily in concentrated hydrochloric, hydroiodic and perchloric acids.[29] Molten metal must be kept in a vacuum or an inert atmosphere to avoid reaction with air.[14] At 135 °C the metal will ignite in air and will explode if placed in carbon tetrachloride.[30]

Plutonium is a reactive metal. In moist air or moist argon, the metal oxidizes rapidly, producing a mixture of oxides and hydrides.[4] If the metal is exposed long enough to a limited amount of water vapor, a powdery surface coating of PuO2 is formed.[4] Also formed is plutonium hydride but an excess of water vapor forms only PuO2.[29]
With this coating, the metal is pyrophoric, meaning it can ignite spontaneously, so plutonium metal is usually handled in an inert, dry atmosphere of nitrogen or argon. Oxygen retards the effects of moisture and acts as a passivating agent.[4]
Plutonium shows enormous, and reversible, reaction rates with pure hydrogen, forming plutonium hydride.[10] It also reacts readily with oxygen, forming PuO and PuO2 as well as intermediate oxides; plutonium oxide fills 40% more volume than plutonium metal. It reacts with the halogens, giving rise to compounds such as PuX3 where X can be F, Cl, Br or I; PuF4 is also seen. The following oxyhalides are observed: PuOCl, PuOBr and PuOI. It will react with carbon to form PuC, nitrogen to form PuN and silicon to form PuSi2.[13][30]
Crucibles used to contain plutonium need to be able to withstand its strongly reducing properties. Refractory metals such as tantalum and tungsten along with the more stable oxides, borides, carbides, nitrides and silicides can tolerate this. Melting in an electric arc furnace can be used to produce small ingots of the metal without the need for a crucible.[14]
Cerium is used as a chemical simulant of plutonium for development of containment, extraction, and other technologies.[31]
Electronic structure: 5f electrons
The anomalous behavior of plutonium is caused by its electronic structure. The energy difference between the 6d and 5f subshells is very low. The size of the 5f shell is just enough to allow the electrons to form bonds within the lattice, on the very boundary between localized and bonding behavior. The proximity of energy levels leads to multiple low-energy electron configurations with near equal energy levels. This leads to competing 5fn7s2 and 5fn-17s26d1 configurations, which causes the complexity of its chemical behavior. The highly directional nature of 5f orbitals is responsible for directional covalent bonds in molecules and complexes of plutonium.[10]
Alloys
Plutonium can form alloys and intermediate compounds with most other metals. Exceptions include lithium, sodium, potassium, and rubidium of the alkali metals; and magnesium, calcium, strontium, and barium of the alkaline earth metals; and europium and ytterbium of the rare earth metals.[29] Partial exceptions include the refractory metals chromium, molybdenum, niobium, tantalum, and tungsten, which are soluble in liquid plutonium, but insoluble or only slightly soluble in solid plutonium.[29] Gallium, aluminium, americium, scandium and cerium can stabilize the δ phase of plutonium for room temperature. Silicon, indium, zinc and zirconium allow formation of metastable δ state when rapidly cooled. High amounts of hafnium, holmium and thallium also allows retaining some of the δ phase at room temperature. Neptunium is the only element that can stabilize the α phase at higher temperatures.[10]
Plutonium alloys can be produced by adding a metal to molten plutonium. However, if the alloying metal is sufficiently reductive, plutonium can be added in the form of oxides or halides. The δ phase plutonium-gallium and plutonium-aluminium alloys are produced by adding plutonium(III) fluoride to molten gallium or aluminium, which has the advantage of avoiding dealing directly with the highly reactive plutonium metal.[32]
- Plutonium-gallium is used for stabilizing the δ phase of plutonium, avoiding the α-phase and α-δ related issues. Its main use is in pits of implosion nuclear weapons.[33]
- Plutonium-aluminium is an alternative to the Pu-Ga alloy. It was the original element considered for δ phase stabilization, however its tendency to react with the alpha particles and release neutrons reduces its usability for nuclear weapon pits. Plutonium-aluminium alloy can be also used as a component of nuclear fuel.[34]
- Plutonium-gallium-cobalt alloy (PuCoGa5) is an unconventional superconductor, showing superconductivity below 18.5 kelvin, an order of magnitude higher than the highest between heavy fermion systems, and has large critical current.[16][35]
- Plutonium-zirconium alloy can be used as nuclear fuel.[36]
- Plutonium-cerium and plutonium-cerium-cobalt alloys are used as nuclear fuels.[37]
- Plutonium-uranium, with about 15-30 mol.% plutonium, can be used as a nuclear fuel for fast breeder reactors. However its pyrophoric nature and high susceptibility to corrosion to the point of self-igniting or disintegrating after exposure to air requires alloying with other components. Addition of aluminium, carbon or copper did not improve disintegration rates markedly, zirconium and iron alloys have better corrosion resistance but they disintegrate in several months in air as well. Addition of titanium and/or zirconium significantly increases the melting point of the alloy.[38]
- Plutonium-uranium-titanium and plutonium-uranium-zirconium were investigated for use as nuclear fuels. The addition of the third element increases corrosion resistance, reduces flammability, and improves ductility, fabricability, strength, and thermal expansion. Plutonium-uranium-molybdenum has the best corrosion resistance, forming a protective film of oxides, but titanium and zirconium are preferred for physics reasons.[38]
- Thorium-uranium-plutonium was investigated as a nuclear fuel for fast breeder reactors.[38]
Occurrence
Trace amounts of at least two plutonium isotopes (plutonium-239 and 244) can be found in nature. Small traces of plutonium-239, a few parts per trillion, and its decay products are naturally found in some concentrated ores of uranium,[39] such as the natural nuclear fission reactor in Oklo, Gabon.[40] The ratio of plutonium-239 to uranium at the Cigar Lake Mine uranium deposit ranges from 2.4 × 10−12 to 44 × 10−12.[41] Even smaller amounts of primordial plutonium-244 occur naturally due to its relatively long half-life of about 80 million years.[42] These trace amounts of Pu-239 originate in the following fashion: On rare occasions, U-238 undergoes spontaneous fission, and in the process, the nucleus emits one or two free neutrons with some kinetic energy. When one of these neutrons strikes the nucleus of another U-238 atom, it is absorbed and the atom, which becomes U-239. With quite-short half-lives, U-239 decays to neptunium-239 (Np-239), and then Np-239 decays into Pu-239.
Since the relatively long-lived isotope plutonium-240 occurs in the decay chain of plutonium-244 it should also be present, albeit 10,000 times rarer still. Finally, exceedingly small amounts of plutonium-238, attributed to the incredibly rare double beta decay of uranium-238, have been found in natural uranium samples.[43]
Minute traces of plutonium are usually found in the human body due to the 550 atmospheric and underwater nuclear tests that have been carried and a small number of major nuclear accidents. Most atmospheric and underwater nuclear testing was stopped by the Limited Test Ban Treaty in 1963, which was signed and ratified by the United States, the United Kingdom, the Soviet Union, and other nations. Continued atmospheric nuclear weapons testing since 1963 by non-treaty nations included those by China (atomic bomb test above the Gobi Desert in 1964, hydrogen bomb test in 1967, and follow-on tests), and France (tests as recently as the 1980s).
Because it is purposely manufactured for nuclear weapons and nuclear reactors, plutonium-239 is the most abundant isotope of plutonium by far.[30]
It is also hypothetically possible for minute quantities of plutonium to be produced by the natural bombardment of uranium ores with cosmic rays.
History
Discovery
Enrico Fermi and a team of scientists at the University of Rome reported that they had discovered element 94 in 1934.[44] Fermi called the element hesperium and mentioned it in his Nobel Lecture in 1938.[45] The sample was actually a mixture of barium, krypton, and other elements, but this was not known at the time because nuclear fission had not been discovered yet.[46]

Plutonium (specifically, plutonium-238) was first produced and isolated on December 14, 1940, and chemically identified on February 23, 1941, by Dr. Glenn T. Seaborg, Edwin M. McMillan, J. W. Kennedy, and A. C. Wahl by deuteron bombardment of uranium in the 60-inch (150 cm) cyclotron at the University of California, Berkeley.[47] In the 1940 experiment, neptunium-238 was created directly by the bombardment but decayed by beta emission two days later, which indicated the formation of element 94.[30]
A paper documenting the discovery was prepared by the team and sent to the journal Physical Review in March 1941.[30] The paper was withdrawn before publication after the discovery that an isotope of the new element (plutonium-239) could undergo nuclear fission in a way that might be useful in an atomic bomb. Publication was delayed until a year after the end of World War II due to security concerns.[17]
Edwin McMillan had recently named the first transuranium element after the planet Neptune and suggested that element 94, being the next element in the series, be named for what was then considered the next planet, Pluto.[7][note 2] Seaborg originally considered the name "plutium", but later thought that it did not sound as good as "plutonium."[48] He chose the letters "Pu" as a joke, which passed without notice into the periodic table.[note 3] Alternate names considered by Seaborg and others were "ultimium" or "extremium" because of the erroneous belief that they had found the last possible element on the periodic table.[49]
Early research
The basic chemistry of plutonium was found to resemble uranium after a few months of initial study.[30] Early research was continued at the secret Metallurgical Laboratory of the University of Chicago. On August 18, 1942, a trace quantity of this element was isolated and measured for the first time. About 50 micrograms of plutonium-239 combined with uranium and fission products was produced and only about 1 microgram was isolated.[39] This procedure enabled chemists to determine the new element's atomic weight.[50][note 4]
In November 1943 some plutonium trifluoride was reduced to create the first sample of plutonium metal: a few micrograms of metallic beads.[39] Enough plutonium was produced to make it the first synthetically made element to be visible with the unaided eye.[51]
The nuclear properties of plutonium-239 were also studied; researchers found that when it is hit by a neutron it breaks apart (fissions) by releasing more neutrons and energy. These neutrons can hit other atoms of plutonium-239 and so on in an exponentially fast chain reaction. This can result in an explosion large enough to destroy a city if enough of the isotope is concentrated to form a critical mass.[30]
Production during the Manhattan Project
During World War II the U.S. government established the Manhattan Project, which was tasked with developing an atomic bomb. The three primary research and production sites of the project were the plutonium production facility at what is now the Hanford Site, the uranium enrichment facilities at Oak Ridge, Tennessee, and the weapons research and design laboratory, now known as Los Alamos National Laboratory.[52]

The first production reactor that made plutonium-239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the Oak Ridge National Laboratory.[30][note 5]
On April 5, 1944, Emilio Segrè at Los Alamos received the first sample of reactor-produced plutonium from Oak Ridge.[53] Within ten days, he discovered that reactor-bred plutonium had a higher concentration of the isotope plutonium-240 than cyclotron-produced plutonium. Plutonium-240 has a high spontaneous fission rate, raising the overall background neutron level of the plutonium sample. The original gun-type plutonium weapon, code-named "Thin Man", had to be abandoned as a result—the increased number of spontaneous neutrons meant that nuclear pre-detonation (a fizzle) would be likely.
The entire plutonium weapon design effort at Los Alamos was soon changed to the more complicated implosion device, code-named "Fat Man." With an implosion weapon, a solid (or, in later designs, hollow) sphere of plutonium is compressed to a high density with explosive lenses—a technically more daunting task than the simple gun-type design, but necessary in order to use plutonium for weapons purposes. (Enriched uranium, by contrast, can be used with either method.)[53]
Construction of the Hanford B Reactor, the first industrial-sized nuclear reactor for the purposes of material production, was completed in March 1945. B Reactor produced the fissile material for the plutonium weapons used during World War II.[note 6] B, D and F were the initial reactors built at Hanford, and six additional plutonium-producing reactors were built later at the site.[54]
In 2004, a safe was discovered during excavations of a burial trench at the Hanford nuclear site. Inside the safe were various items, including a large glass bottle containing a whitish slurry which was subsequently identified as the oldest sample of weapons-grade plutonium known to exist. Isotope analysis by Pacific Northwest National Laboratory indicated that the plutonium in the bottle was manufactured in the X-10 reactor at Oak Ridge during 1944. [55][56][57]
Trinity and Fat Man atomic bombs
The first atomic bomb test, codenamed "Trinity" and detonated on July 16, 1945, near Alamogordo, New Mexico, used plutonium as its fissile material.[39] The implosion design of "the Gadget", as the Trinity device was code-named, used conventional explosive lenses to compress a sphere of plutonium into a supercritical mass, which was simultaneously showered with neutrons from the "Urchin", an initiator made of polonium and beryllium (neutron source: (α, n) reaction).[30] Together, these ensured a runaway chain reaction and explosion. The overall weapon weighed over 4 tonnes, although it used just 6.2 kg of plutonium in its core.[58] About 20% of the plutonium used in the Trinity weapon underwent fission, resulting in an explosion with an energy equivalent to approximately 20,000 tons of TNT.[59][note 7]
An identical design was used in the "Fat Man" atomic bomb dropped on Nagasaki, Japan, on August 9, 1945, killing 70,000 people and wounding another 100,000.[30] The "Little Boy" bomb dropped on Hiroshima three days earlier used uranium-235, not plutonium. Japan capitulated on August 15 to General Douglas MacArthur. Only after the announcement of the first atomic bombs was the existence of plutonium made public.
Cold War use and waste
Large stockpiles of weapons-grade plutonium were built up by both the Soviet Union and the United States during the Cold War. The U.S. reactors at Hanford and the Savannah River Site in South Carolina produced 103 tonnes,[60] and an estimated 170 tonnes of military-grade plutonium was produced in Russia.[61][note 8] Each year about 20 tonnes of the element is still produced as a by-product of the nuclear power industry.[13] As much as 1000 tonnes of plutonium may be in storage with more than 200 tonnes of that either inside or extracted from nuclear weapons.[30] SIPRI estimated the world plutonium stockpile in 2007 as about 500 tons, divided equally between weapon and civilian stocks.[62]
Since the end of the Cold War, these stockpiles have become a focus of nuclear proliferation concerns. In the U.S., some plutonium extracted from dismantled nuclear weapons is melted to form glass logs of plutonium oxide that weigh two tonnes.[30] The glass is made of borosilicates mixed with cadmium and gadolinium.[note 9] These logs are planned to be encased in stainless steel and stored as much as 4 km underground in bore holes that will be back-filled with concrete.[30] As of 2008, the only facility in the U.S. that is scheduled to store plutonium in this way is the Yucca Mountain nuclear waste repository, which is about 100 miles (160 km) north-east of Las Vegas, Nevada.[63] Local and state opposition to this plan has delayed efforts to store nuclear waste at Yucca Mountain.
Medical experimentation
During and after the end of World War II, scientists working on the Manhattan Project and other nuclear weapons research projects conducted studies of the effects of plutonium on laboratory animals and human subjects.[64] Animal studies found that a few milligrams of plutonium per kilogram of tissue is a lethal dose.[65]
In the case of human subjects, this involved injecting solutions containing (typically) five micrograms of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease condition.[64] This was reduced to one microgram in July 1945 after animal studies found that the way plutonium distributed itself in bones was more dangerous than radium.[65]
Eighteen human test subjects were injected with plutonium without informed consent. The tests were used to create diagnostic tools to determine the uptake of plutonium in the body in order to develop safety standards for working with plutonium.[64]
The episode is now considered to be a serious breach of medical ethics and of the Hippocratic Oath. More sympathetic commentators have noted that while it was definitely a breach in trust and ethics, "the effects of the plutonium injections were not as damaging to the subjects as the early news stories painted, nor were they so inconsequential as many scientists, then and now, believe."[66]
Applications
Explosives

The isotope plutonium-239 is a key fissile component in nuclear weapons, due to its ease of fission and availability. Encasing the bomb's plutonium pit in a tamper (an optional layer of dense material) decreases the amount of plutonium needed to reach critical mass by reflecting escaping neutrons back into the plutonium core. This reduces the amount of plutonium needed to reach criticality from 16 kg to 10 kg, which is a sphere with a diameter of about 10 centimeters (4 in).[67] This critical mass is about a third of that for uranium-235.[7]
The "Fat Man"-type plutonium bombs produced during the Manhattan Project used explosive compression of plutonium to obtain significantly higher densities than normal, combined with a central neutron source to begin the reaction and increase efficiency. Thus only 6.2 kg of plutonium was needed for an explosive yield equivalent to 20 kilotons of TNT.[59][68] (See also Nuclear weapon design.) Hypothetically, as little as 4 kg of plutonium—and maybe even less—could be used to make a single atomic bomb using very sophisticated assembly designs.[68]
Use of nuclear waste
Spent nuclear fuel from normal light water reactors contains Plutonium, but it is a mixture of Plutonium-242, 240, 239 and 238. The mixture is not sufficiently enriched for efficient nuclear weapons, but can be used once as MOX fuel. Accidental neutron capture causes the amount of Plutonium-242 and 240 to grow each time the Plutonium is irradiated in a reactor with low-speed "thermal" neutrons, so that after the second cycle, the Plutonium can only be consumed by fast neutron reactors. If fast neutron reactors are not available (the normal case), excess Plutonium is usually discarded, and forms the longest-lived component of nuclear waste. The desire to consume this Plutonium and other transuranic fuels and reduce the radiotoxicity of the waste is the usual reason nuclear engineers give to make fast neutron reactors.
The most common chemical process, PUREX (Plutonium–URanium EXtraction) reprocesses spent nuclear fuel to extract plutonium and uranium to form a mixed oxide "MOX fuel" for reuse in nuclear reactors. Weapons grade plutonium can be added to the fuel mix. MOX fuel is used in light water reactors and consists of 60 kg of plutonium per tonne of fuel; after four years, three-quarters of the plutonium is burned (turned into other elements).[30] Breeder reactors are specifically designed to create more fissionable material than they consume.
MOX fuel has been in use since the 1980s and is widely used in Europe.[69] In September 2000, the United States and the Russian Federation signed a Plutonium Management and Disposition Agreement by which each agreed to dispose of 34 tonnes of weapon grade plutonium.[70] The U.S. Department of Energy plans to dispose of 34 tonnes of weapon grade plutonium in the United States before the end of 2019 by converting the plutonium to a MOX fuel to be used in commercial nuclear power reactors.[70]
MOX fuel improves total burnup. A fuel rod is reprocessed after three years of use to remove waste products, which by then account for 3% of the total weight of the rods.[30] Any uranium or plutonium isotopes produced during those three years are left and the rod goes back into production.[note 10] However, the presence of up to 1% gallium per mass in weapon grade plutonium alloy has the potential to interfere with long-term operation of a light water reactor.[71]
Plutonium recovered from spent reactor fuel is not a significant proliferation hazard, because of excessive contamination with non-fissile plutonium-240 and plutonium-242. Separation of the isotopes is not feasible. A dedicated reactor operating on very low burnup is generally required to produce material suitable for use in nuclear weapons.[72] 241Am has recently been suggested for use as a denaturing agent in plutonium reactor fuel rods to further limit its proliferation potential.[73]
Power and heat source

The isotope plutonium-238 has a half-life of 87.74 years.[74] It emits a large amount of thermal energy with low levels of both gamma rays/particles and spontaneous neutron rays/particles.[75] Being an alpha emitter, it combines high energy radiation with low penetration and thereby requires minimal shielding. A sheet of paper can be used to shield against the alpha particles emitted by plutonium-238 while one kilogram of the isotope can generate about 570 watts of heat energy.[7][75]
These characteristics make it well-suited for electrical power generation for devices which must function without direct maintenance for timescales approximating a human lifetime. It is therefore used in radioisotope thermoelectric generators and radioisotope heater units such as those in the Cassini, Voyager and New Horizons space probes.
The twin Voyager spacecraft were launched in 1977 with each containing a 500 watt plutonium power source. Over 30 years later each source is still producing about 300 watts which allows limited operation of each spacecraft.[76] Earlier versions of the same technology powered the ALSEP and EASEP systems including seismic experiments on the Apollo 14 Moon mission.[30]
Plutonium-238 has also been used successfully to power artificial heart pacemakers, to reduce the risk of repeated surgery.[77][78] It has been largely replaced by lithium-based primary cells, but as of 2003 there were somewhere between 50 and 100 plutonium-powered pacemakers still implanted and functioning in living patients.[79] Plutonium-238 was studied as way to provide supplemental heat to scuba diving.[80] Plutonium-238 mixed with beryllium is used to generate neutrons for research purposes.[30]
Precautions
Toxicity
Isotopes and compounds of plutonium are dangerous due to their radioactivity. Contamination by plutonium oxide (spontaneously oxidized plutonium) has resulted from a number of military nuclear accidents where nuclear weapons have burned.[81] However, based on chemical toxicity alone, the element is less dangerous than arsenic or cyanide and about the same as caffeine.[82][83]
The alpha radiation plutonium emits does not penetrate the skin but can irradiate internal organs when plutonium is inhaled or ingested.[30] The skeleton, where plutonium is absorbed by the bone surface, and the liver, where it collects and becomes concentrated, are at risk.[29]. Plutonium is not absorbed into the body efficiently when ingested; only 0.04% of plutonium oxide is absorbed after ingestion.[30] What plutonium is absorbed into the body is excreted very slowly, with a biological half-life of 200 years.[84] Plutonium passes only slowly through cell membranes and intestinal boundaries, so absorption by ingestion and incorporation into bone structure proceeds very slowly.[85][86]
Plutonium is more dangerous when inhaled than when ingested. The risk of lung cancer increases once the total dose equivalent of inhaled radiation exceeds 400 mSv.[87] The U.S. Department of Energy estimates that the lifetime cancer risk for inhaling 5,000 plutonium particles, each about 3 microns wide, to be 1% over the background U.S. average.[88] Ingestion or inhalation of large amounts may cause acute radiation poisoning and death, however no human is known to have died because of inhaling or ingesting plutonium, and many people have measurable amounts of plutonium in their bodies.[83]
The "hot particle" theory in which a particle of plutonium dust radiates a localized spot of lung tissue has been tested and found false – such particles are more mobile than originally thought and toxicity is not measurably increased due to particulate form.[85] Several populations of people who have been exposed to plutonium dust (e.g. people living down-wind of Nevada test sites, Hiroshima survivors, nuclear facility workers, and "terminally ill" patients injected with Pu in 1945-46 to study Pu metabolism) have been carefully followed and analyzed.
These studies generally do not show especially high plutonium toxicity or plutonium-induced cancer results.[85] "There were about 25 workers from Los Alamos National Laboratory who inhaled a considerable amount of plutonium dust during the 1940's; according to the hot-particle theory, each of them has a 99.5% chance of being dead from lung cancer by now, but there has not been a single lung cancer among them."[89][90]
Plutonium has a metallic taste.[91]
Criticality potential

Toxicity issues aside, care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium-235.[7] A critical mass of plutonium emits lethal amounts of neutrons and gamma rays.[92] Plutonium in solution is more likely to form a critical mass than the solid form due to moderation by the hydrogen in water.[13]
Criticality accidents have occurred in the past, some of them with lethal consequences. Careless handling of tungsten carbide bricks around a 6.2 kg plutonium sphere resulted in a fatal dose of radiation at Los Alamos on August 21, 1945, when scientist Harry K. Daghlian, Jr. received a dose estimated to be 5.1 Sievert (510 rems) and died 28 days later.[93] Nine months later, another Los Alamos scientist, Louis Slotin, died from a similar accident involving a beryllium reflector and the same plutonium core (the so-called "demon core") that had previously claimed the life of Daghlian.[94] These incidents were fictionalized in the 1989 film Fat Man and Little Boy.
In December 1958, during a process of purifying plutonium at Los Alamos, a critical mass was formed in a mixing vessel, which resulted in the death of a crane operator.[95] Other nuclear accidents have occurred in the Soviet Union, Japan, and many other countries.[95]
Flammability
Metallic plutonium is a fire hazard, especially if the material is finely divided. In a moist environment, plutonium forms hydrides on its surface, which are pyrophoric and may ignite in air at room temperature. Plutonium expands up to 70% in volume as it oxidizes and thus may break its container.[96] The radioactivity of the burning material is an additional hazard. Magnesium oxide sand is probably the most effective material for extinguishing a plutonium fire. It cools the burning material, acting as a heat sink, and also blocks off oxygen. Special precautions are necessary to store or handle plutonium in any form; generally a dry inert gas atmosphere is required.[96][97][note 11]
See also
- Nuclear engineering
- Nuclear fuel cycle
- Nuclear physics
Notes
- ↑ The PuO2+ ion is unstable in solution and will disproportionate into Pu4+ and PuO22+; the Pu4+ will then oxidize the remaining PuO2+ to PuO22+, being reduced in turn to Pu3+. Thus, aqueous solutions of plutonium tend over time towards a mixture of Pu3+ and PuO22+.
- Crooks, William J. (2002). "Nuclear Criticality Safety Engineering Training Module 10 – Criticality Safety in Material Processing Operations, Part 1" (PDF). http://ncsp.llnl.gov/ncset/Module10.pdf. Retrieved 2006-02-15.
- ↑ This was not the first time somebody suggested that an element be named "plutonium." A decade after barium was discovered, a Cambridge University professor suggested it be renamed to "plutonium" because the element was not (as suggested by the Greek root, barys, it was named for) heavy. He reasoned that, since it was produced by the relatively new technique of electrolysis, its name should refer to fire. Thus he suggested it be named for the Roman god of the underworld, Pluto. (Heiserman 1992)
- ↑ As one article puts it, referring to information Seaborg gave in a talk: "The obvious choice for the symbol would have been Pl, but facetiously, Seaborg suggested Pu, like the words a child would exclaim, 'Pee-yoo!' when smelling something bad. Seaborg thought that he would receive a great deal of flak over that suggestion, but the naming committee accepted the symbol without a word."
- Clark, David L.; Hobart, David E. (2000). "Reflections on the Legacy of a Legend: Glenn T. Seaborg, 1912–1999" (PDF). Los Alamos Science 26: 56–61, on 57. http://www.fas.org/sgp/othergov/doe/lanl/pubs/00818011.pdf. Retrieved 2009-02-15.
- ↑ Room 405 of the George Herbert Jones Laboratory, where the first isolation of plutonium took place, was named a National Historic Landmark in May 1967.
- ↑ During the Manhattan Project, plutonium was also often referred to as simply "49": the number 4 was for the last digit in 94 (atomic number of plutonium), and 9 was for the last digit in plutonium-239, the weapon-grade fissile isotope used in nuclear bombs.
- Hammel, E.F. (2000). "The taming of "49" – Big Science in little time. Recollections of Edward F. Hammel, pp. 2-9. In: Cooper N.G. Ed. (2000). Challenges in Plutonium Science". Los Alamos Science 26 (1): 2–9. http://www.fas.org/sgp/othergov/doe/lanl/pubs/00818010.pdf. Retrieved 2009-02-15.
- Hecker, S.S. (2000). "Plutonium: an historical overview. In: Challenges in Plutonium Science". Los Alamos Science 26 (1): 1–2. http://www.fas.org/sgp/othergov/doe/lanl/pubs/number26.htm. Retrieved 2009-02-15.
- ↑ The American Society of Mechanical Engineers (ASME) established B Reactor as a National Historic Mechanical Engineering Landmark in September 1976.
- Wahlen, R.K. (1989) (PDF). History of 100-B Area. Richland, Washington: Westinghouse Hanford Company. p. 1. WHC-EP-0273. http://www.hanford.gov/doe/history/files/HistoryofBArea.pdf. Retrieved 2009-02-15.
- "Weekly List Actions". National Park Service. 2008-08-29. http://www.nps.gov/history/nr/listings/20080829.HTM. Retrieved 2008-08-30.
- ↑ The efficiency calculation is based on the fact that 1 kg of plutonium-239 (or uranium-235) fissioning results in an energy release of approximately 17 kt, leading to a rounded estimate of 1.2 kg plutonium actually fissioned to produce the 20 kt yield. On the figure of 1 kg = 17 kt,
- Garwin, Richard (2002-10-04). "Proliferation of Nuclear Weapons and Materials to State and Non-State Actors: What It Means for the Future of Nuclear Power". University of Michigan Symposium. Federation of American Scientists. http://www.fas.org/rlg/PNWM_UMich.pdf. Retrieved 2009-01-04.
- ↑ Much of this plutonium was used to make the fissionable cores of a type of thermonuclear weapon employing the Teller–Ulam design. These so-called 'hydrogen bombs' are a variety of nuclear weapon that use a fission bomb to trigger the nuclear fusion of heavy hydrogen isotopes. Their destructive yield is commonly in the millions of tons of TNT equivalent compared with the thousands of tons of TNT equivalent of fission-only devices.(Emsley 2001)
- ↑ Gadolinium zirconium oxide (Gd2Zr2O7) has been studied because it could hold plutonium for up to 30 million years.(Emsley 2001)
- ↑ Breakdown of plutonium in a spent nuclear fuel rod: plutonium-239 (~58%), 240 (24%), 241 (11%), 242 (5%), and 238 (2%). (Emsley 2001)
- ↑ There was a major plutonium-initiated fire at the Rocky Flats Plant near Boulder, Colorado in 1969.
- Albright, David; O'Neill, Kevin (1999). "The Lessons of Nuclear Secrecy at Rocky Flats". ISIS Issue Brief. Institute for Science and International Security (ISIS). http://www.isis-online.org/publications/usfacilities/Rfpbrf.html. Retrieved 2008-12-07.
References
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ BNL-NCS 51363, vol. II (1981), pages 835ff
- ↑ "'Detection of Plutonium-244 in Nature,' Nature 234, 132-134 (19 November 1971)" (PDF). http://www.nature.com/nature/journal/v234/n5325/abs/234132a0.html.
- ↑ 4.0 4.1 4.2 4.3 4.4 NIH contributors. "Plutonium, Radioactive". Wireless Information System for Emergency Responders (WISER). Bethesda (MD): U.S. National Library of Medicine, National Institutes of Health. http://webwiser.nlm.nih.gov/getSubstanceData.do;jsessionid=89B673C34252C77B4C276F2B2D0E4260?substanceID=419&displaySubstanceName=Plutonium,%20Radioactive&UNNAID=&STCCID=&selectedDataMenuItemID=44. Retrieved 2008-11-23. (public domain text)
- ↑ ARQ staff (2008). "Nitric acid processing". Actinide Research Quarterly (Los Alamos (NM): Los Alamos National Laboratory) (3rd quarter). http://arq.lanl.gov/source/orgs/nmt/nmtdo/AQarchive/3rdQuarter08/page3.shtml. Retrieved 2010-02-09. "While plutonium dioxide is normally olive green, samples can be various colors. It is generally believed that the color is a function of chemical purity, stoichiometry, particle size, and method of preparation, although the color resulting from a given preparation method is not always reproducible.".
- ↑ 6.0 6.1 6.2 NNDC contributors; Alejandro A. Sonzogni (Database Manager) (2008). "Chart of Nuclides". Upton (NY): National Nuclear Data Center, Brookhaven National Laboratory. http://www.nndc.bnl.gov/chart/. Retrieved 2008-09-13.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9 Heiserman 1992
- ↑ Rhodes, Richard (1986). The Making of the Atomic Bomb. New York: Simon & Schuster. pp. 659–660. ISBN 0-671-65719-4. Leona Marshall: "When you hold a lump of it in your hand, it feels warm, like a live rabbit"
- ↑ 9.0 9.1 9.2 9.3 Miner 1968, p. 544
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 Hecker, Siegfried S. (2000). "Plutonium and its alloys: from atoms to microstructure" (PDF). Los Alamos Science 26: 290–335. http://www.fas.org/sgp/othergov/doe/lanl/pubs/00818035.pdf. Retrieved 2009-02-15.
- ↑ Hecker, Siegfried S.; Martz, Joseph C. (2000). "Aging of Plutonium and Its Alloys" (PDF). Los Alamos Science (Los Alamos, New Mexico: Los Alamos National Laboratory) (26): 242. http://library.lanl.gov/cgi-bin/getfile?00818029.pdf. Retrieved 2009-02-15.
- ↑ 12.0 12.1 12.2 12.3 Baker, Richard D.; Hecker, Siegfried S.; Harbur, Delbert R. (1983). "Plutonium: A Wartime Nightmare but a Metallurgist's Dream". Los Alamos Science (Los Alamos National Laboratory): 148, 150–151. http://library.lanl.gov/cgi-bin/getfile?07-16.pdf. Retrieved 2009-02-15.
- ↑ 13.0 13.1 13.2 13.3 13.4 CRC 2006, p. 4–27
- ↑ 14.0 14.1 14.2 14.3 Miner 1968, p. 542
- ↑ "Plutonium Crystal Phase Transitions". GlobalSecurity.org. http://www.globalsecurity.org/wmd/intro/pu-phase.htm.
- ↑ 16.0 16.1 Dumé, Belle (November 20, 2002). "Plutonium is also a superconductor". PhysicsWeb.org. http://physicsworld.com/cws/article/news/16443.
- ↑ 17.0 17.1 Stwertka 1998
- ↑ EPA contributors (2008). "Fissile Material". Radiation Glossary. United States Environmental Protection Agency. http://www.epa.gov/rpdweb00/glossary/termdef.html#f. Retrieved 2008-11-23.
- ↑ Asimov, Isaac (1988). "Nuclear Reactors". Understanding Physics. Barnes & Noble Publishing. p. 905. ISBN 0880292512.
- ↑ Samuel Glasstone and Leslie M. Redman, An Introduction to Nuclear Weapons (Atomic Energy Commission Division of Military Applications Report WASH-1038, June 1972), p. 12.
- ↑ Gosling, F.G. (1999). The Manhattan Project: Making the Atomic Bomb. Oak Ridge (TN): United States Department of Energy. p. 40. DOE/MA-0001-01/99. http://www.cfo.doe.gov/me70/manhattan/publications/DE99001330.pdf. Retrieved 2009-02-15.
- ↑ DOE contributors (1996). Plutonium: The First 50 Years. U.S. Department of Energy. DOE/DP-1037. http://www.doeal.gov/SWEIS/DOEDocuments/004%20DOE-DP-0137%20Plutonium%2050%20Years.pdf. (public domain text)
- ↑ Kennedy, J. W.; Seaborg, G. T.; Segrè, E.; Wahl, A. C. (1946). "Properties of Element 94". Physical Review 70 (7–8): 555–556. doi:10.1103/PhysRev.70.555.
- ↑ Greenwood 1997, p. 1259
- ↑ Seaborg, Glenn T.; McMillan, E.; Kennedy, J. W.; Wahl, A. C. (1946). "Radioactive Element 94 from Deuterons on Uranium". Physical Review 69 (7–8): 366–367. doi:10.1103/PhysRev.69.367.
- ↑ "Can Reactor Grade Plutonium Produce Nuclear Fission Weapons?". Council for Nuclear Fuel Cycle Institute for Energy Economics, Japan. May 2001. http://www.cnfc.or.jp/e/proposal/reports/index.html.
- ↑ Matlack, George (2002). A Plutonium Primer: An Introduction to Plutonium Chemistry and its Radioactivity. Los Alamos National Laboratory. LA-UR-02-6594.
- ↑ Eagleson, Mary (1994). Concise Encyclopedia Chemistry. Walter de Gruyter. p. 840. ISBN 9783110114515.
- ↑ 29.0 29.1 29.2 29.3 29.4 Miner 1968, p. 545
- ↑ 30.00 30.01 30.02 30.03 30.04 30.05 30.06 30.07 30.08 30.09 30.10 30.11 30.12 30.13 30.14 30.15 30.16 30.17 30.18 Emsley 2001
- ↑ Crooks, W. J. et al. (2002). "Low Temperature Reaction of ReillexTM HPQ and Nitric Acid". Solvent Extraction and Ion Exchange 20: 543. doi:10.1081/SEI-120014371. http://sti.srs.gov/fulltext/ms2000068/ms2000068.html.
- ↑ Moody, Kenton James; Hutcheon, Ian D.; Grant, Patrick M. (2005). "plutonium+alloys"&cd=22#v=onepage&q=%22plutonium%20alloys%22 Nuclear forensic analysis. CRC Press. p. 169. ISBN 0849315131. http://books.google.com/?id=W3FnEOg8tS4C&pg=PA169&dq="plutonium+alloys"&cd=22#v=onepage&q=%22plutonium%20alloys%22.
- ↑ Kolman, D. G. and Colletti, L. P. (2009). "The aqueous corrosion behavior of plutonium metal and plutonium-gallium alloys exposed to aqueous nitrate and chloride solutions". ECS transactions. 16. Electrochemical Society. p. 71. ISSN 1938-5862. http://books.google.com/?id=0o4DnYptWdgC&pg=PA71.
- ↑ Hurst, D. G. and Ward, A. G.. Canadian Research Reactors. Los Alamos National Laboratory. http://www.csirc.net/docs/reports/ref_066.pdf.
- ↑ Curro, N. J. (Spring 2006). Unconventional superconductivity in PuCoGa5. Los Alamos National Laboratory. http://www.lanl.gov/orgs/mpa/files/mrhighlights/LALP-06-072.pdf.
- ↑ McCuaig, Franklin D. "Pu-Zr alloy for high-temperature foil-type fuel" U.S. Patent 4,059,439, Issued on November 22, 1977
- ↑ Jha, D.K. (2004). Nuclear Energy. Discovery Publishing House. p. 73. ISBN 8171418848. http://books.google.com/?id=L79odes2ihEC&pg=PA73.
- ↑ 38.0 38.1 38.2 plutonium 1965. Taylor & Francis. 1965. p. 456. http://books.google.com/?id=8r8NAAAAQAAJ&pg=PA456.
- ↑ 39.0 39.1 39.2 39.3 Miner 1968, p. 541
- ↑ DOE contributors (2004). "Oklo: Natural Nuclear Reactors". U.S. Department of Energy, Office of Civilian Radioactive Waste Management. http://www.ocrwm.doe.gov/factsheets/doeymp0010.shtml. Retrieved 2008-11-16.
- ↑ Curtis, David; Fabryka-Martin, June; Paul, Dixon; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta 63 (2): 275–285. doi:10.1016/S0016-7037(98)00282-8.
- ↑ Hoffman, D. C.; Lawrence, F. O.; Mewherter, J. L.; and Rourke, F. M. (1971). "Detection of Plutonium-244 in Nature". Nature 234: 132–134. doi:10.1038/234132a0. Nr. 34.
- ↑ Peterson, Ivars (December 7, 1991). "Uranium displays rare type of radioactivity". Science News. http://findarticles.com/p/articles/mi_m1200/is_n23_v140/ai_11701241/.
- ↑ Holden, Norman E. (2001). "A Short History of Nuclear Data and Its Evaluation". 51st Meeting of the USDOE Cross Section Evaluation Working Group. Upton (NY): National Nuclear Data Center, Brookhaven National Laboratory. http://www.nndc.bnl.gov/content/evaluation.html. Retrieved 2009-01-03.
- ↑ Fermi, Enrico (December 12, 1938). "Artificial radioactivity produced by neutron bombardment: Nobel Lecture" (PDF). Royal Swedish Academy of Sciences. http://www.nobel.se/physics/laureates/1938/fermi-lecture.pdf.
- ↑ Darden, Lindley (1998). "Enrico Fermi: "Transuranium" Elements, Slow Neutrons". The Nature of Scientific Inquiry. College Park (MD): Department of Philosophy, University of Maryland. http://www.philosophy.umd.edu/Faculty/LDarden/sciinq/. Retrieved 2008-01-03.
- ↑ LBNL contributors. "Elements 93 and 94". Advanced Computing for Science Department, Lawrence Berkeley National Laboratory. http://acs.lbl.gov/Seaborg.talks/65th-anniv/14.html. Retrieved 2008-09-17.
- ↑ Clark, David L.; Hobart, David E. (2000). "Reflections on the Legacy of a Legend: Glenn T. Seaborg, 1912–1999" (PDF). Los Alamos Science 26: 56–61, on 57. http://www.fas.org/sgp/othergov/doe/lanl/pubs/00818011.pdf. Retrieved 2009-02-15.
- ↑ PBS contributors (1997). "Frontline interview with Seaborg". Frontline. Public Broadcasting Service. http://www.pbs.org/wgbh/pages/frontline/shows/reaction/interviews/seaborg.html. Retrieved 2008-12-07.
- ↑ NPS contributors. "Room 405, George Herbert Jones Laboratory". National Park Service. http://tps.cr.nps.gov/nhl/detail.cfm?ResourceId=735&ResourceType=Building. Retrieved 2008-12-14.
- ↑ Miner 1968, p. 540
- ↑ LANL contributors. "Site Selection". LANL History. Los Alamos, New Mexico: Los Alamos National Laboratory. http://www.lanl.gov/history/road/siteselection.shtml. Retrieved 2008-12-23.
- ↑ 53.0 53.1 Sublette, Carey. "Atomic History Timeline 1942-1944". Washington (DC): Atomic Heritage Foundation. http://www.atomicheritage.org/index.php?option=com_content&task=view&id=288&Itemid=202. Retrieved 2008-12-22.
- ↑ Wahlen, R.K. (1989) (PDF). History of 100-B Area. Richland, Washington: Westinghouse Hanford Company. pp. iv, 1. WHC-EP-0273. http://www.hanford.gov/doe/history/files/HistoryofBArea.pdf. Retrieved 2009-02-15.
- ↑ Rincon, Paul (2009-03-02). "BBC NEWS – Science & Environment – US nuclear relic found in bottle". BBC News. http://news.bbc.co.uk/2/hi/science/nature/7918618.stm. Retrieved 2009-03-02.
- ↑ Gebel, Erika (2009). "Old plutonium, new tricks". Analytical Chemistry 81 (5): 1724. doi:10.1021/ac900093b.
- ↑ Schwantes, Jon M.; Matthew Douglas, Steven E. Bonde, James D. Briggs, Orville T. Farmer, Lawrence R. Greenwood, Elwood A. Lepel, Christopher R. Orton, John F. Wacker, Andrzej T. Luksic (2009). "Nuclear archeology in a bottle: Evidence of pre-Trinity U.S. weapons activities from a waste burial site". Analytical Chemistry 81 (4): 1297–1306. doi:10.1021/ac802286a. PMID 19152306.
- ↑ Sublette, Carey (2007-07-03). "8.1.1 The Design of Gadget, Fat Man, and "Joe 1" (RDS-1)". Nuclear Weapons Frequently Asked Questions, edition 2.18. The Nuclear Weapon Archive. http://nuclearweaponarchive.org/Nwfaq/Nfaq8.html#nfaq8.1.1. Retrieved 2008-01-04.
- ↑ 59.0 59.1 Malik, John (September 1985). The Yields of the Hiroshima and Nagasaki Explosions. Los Alamos. p. Table VI. LA-8819. http://www.fas.org/sgp/othergov/doe/lanl/docs1/00313791.pdf. Retrieved 2009-02-15.
- ↑ DOE contributors (2001). Historic American Engineering Record: B Reactor (105-B Building). Richland (WA): U.S. Department of Energy. p. 110. DOE/RL-2001-16. http://www.fas.org/sgp/othergov/doe/pu50yb.html#ZZ13. Retrieved 2008-12-24.
- ↑ Cochran, Thomas B. (1997). "Safeguarding nuclear weapons-usable materials in Russia". International Forum on Illegal Nuclear Traffic. Washington (DC): Natural Resources Defense Council, Inc. http://docs.nrdc.org/nuclear/nuc_06129701a_185.pdf. Retrieved 2008-12-21.
- ↑ Stockholm International Peace Research Institute (2007). SIPRI Yearbook 2007: Armaments, Disarmament, and International Security. Oxford University Press. p. 567. ISBN 0199230218, 9780199230211. http://books.google.com/?id=2M0C6SERFG0C&pg=PA567.
- ↑ Press Secretary (July 23, 2002). "President Signs Yucca Mountain Bill". Washington (DC): Office of the Press Secretary, White House. Archived from the original on 2008-03-06. http://web.archive.org/web/20080306193653/http://georgewbush-whitehouse.archives.gov/news/releases/2002/07/20020723-2.html. Retrieved 2009-01-04.
- ↑ 64.0 64.1 64.2 Moss, William; Eckhardt, Roger (1995). "The Human Plutonium Injection Experiments" (PDF). Los Alamos Science (Los Alamos National Laboratory) 23: 188, 205, 208, 214. http://library.lanl.gov/cgi-bin/getfile?00326640.pdf. Retrieved 2006-06-06.
- ↑ 65.0 65.1 Voelz, George L. (2000). "Plutonium and Health: How great is the risk?". Los Alamos Science (Los Alamos (NM): Los Alamos National Laboratory) (26): 78–79.
- ↑ Yesley, Michael S. (1995). "'Ethical Harm' and the Plutonium Injection Experiments" (PDF). Los Alamos Science 23: 280–283. http://www.fas.org/sgp/othergov/doe/lanl/pubs/00326649.pdf. Retrieved 2009-02-15.
- ↑ Martin, James E. (2000). Physics for Radiation Protection (1st ed.). Wiley-Interscience. p. 532. ISBN 0471353736.
- ↑ 68.0 68.1 FAS contributors (1998). "Nuclear Weapon Design". Federation of American Scientists. http://www.fas.org/nuke/intro/nuke/design.htm. Retrieved 2008-12-07.
- ↑ WNA contributors (2006). "Mixed Oxide Fuel (MOX)". London (UK): World Nuclear Association. http://www.world-nuclear.org/info/inf29.html. Retrieved 2008-12-14.
- ↑ 70.0 70.1 DNFSB staff (2004) (PDF). Plutonium Storage at the Department of Energy's Savannah River Site: First Annual Report to Congress. Defense Nuclear Facilities Safety Board. pp. A–1. Public Law 107-314, Subtitle E. Retrieved 2009-02-15. (public domain text)
- ↑ Besmann, Theodore M. (2005). "Thermochemical Behavior of Gallium in Weapons-Material-Derived Mixed-Oxide Light Water Reactor (LWR) Fuel". Journal of the American Ceramic Society 81 (12): 3071–3076. doi:10.1111/j.1151-2916.1998.tb02740.x.
- ↑ WNA contributors (2009-03). "Plutonium". World Nuclear Association. http://www.world-nuclear.org/info/inf15.html. Retrieved 2010-02-28.
- ↑ "BGU combats nuclear proliferation". March 1, 2010. http://www.jpost.com/HealthAndSci-Tech/ScienceAndEnvironment/Article.aspx?id=134591. Retrieved 2009-03-05.
- ↑ "Science for the Critical Masses: How Plutonium Changes with Time". Institute for Energy and Environmental Research. http://www.ieer.org/ensec/no-3/puchange.html.
- ↑ 75.0 75.1 ARQ contributors (2005). "From heat sources to heart sources: Los Alamos made material for plutonium-powered pumper". Actinide Research Quarterly (Los Alamos (NM): Los Alamos National Laboratory) (1). http://arq.lanl.gov/source/orgs/nmt/nmtdo/AQarchive/05spring/heart.html. Retrieved 2009-02-15.
- ↑ Voyager-Spacecraft Lifetime
- ↑ Venkateswara Sarma Mallela; V. Ilankumaran; and N.Srinivasa Rao (2004). "Trends in Cardiac Pacemaker Batteries". Indian Pacing Electrophysiol 4 (4): 201–212. Full text at PMC: 1502062. PMID 16943934. PMC 1502062. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1502062. Retrieved 2008-12-14.
- ↑ Defunct pacemakers with Pu power source
- ↑ ORAU contributors (1974). "Plutonium Powered Pacemaker". Oak Ridge (TN): Orau.org. http://www.orau.org/PTP/collection/Miscellaneous/pacemaker.htm. Retrieved 2008-09-12.
- ↑ Bayles, John J.; Taylor, Douglas (1970). SEALAB III - Diver's Isotopic Swimsuit-Heater System. Port Hueneme (CA): Naval Civil Engineering Lab. AD0708680. http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=AD0708680.
- ↑ ATSDR contributors (2007). "Toxicological Profile for Plutonium, Draft for Public Comment". U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (ATSDR). http://www.atsdr.cdc.gov/toxprofiles/tp143.html. Retrieved 2008-05-22.
- ↑ Cohen, Bernard L. (1985). Karl Otto Ott and Bernard I. Spinrad, eds. ed. Nuclear Energy (New York (NY): Plenum Press): 355–365.
- ↑ 83.0 83.1 WNA contributors (2008). "Plutonium". London (UK): World Nuclear Association. http://www.world-nuclear.org/info/inf15.html. Retrieved 2008-05-22.
- ↑ DOE staff. "Radiological control technical training". U.S. Department of Energy. Archived from the original on 2007-06-30. http://web.archive.org/web/20070630190114/http://hss.energy.gov/NuclearSafety/techstds/standard/hdbk1122-04/part9of9.pdf. Retrieved 2008-12-14.
- ↑ 85.0 85.1 85.2 Cohen, Bernard L.. "The Myth of Plutonium Toxicity". http://russp.org/BLC-3.html.
- ↑ Cohen, Bernard L. (1977). "Hazards from Plutonium Toxicity". The Radiation Safety Journal: Health Physics (May 32(5)): 359–379.
- ↑ Brown, SC; Schonbeck MF, McClure D et al. (July 2004). "Lung cancer and internal lung doses among plutonium workers at the Rocky Flats Plant: a case-control study". American Journal of Epidemiology (Oxford Journals) 160 (2): 163–172. doi:10.1093/aje/kwh192. PMID 15234938. http://aje.oxfordjournals.org/cgi/content/full/160/2/163. Retrieved 2009-02-15.
- ↑ ANL staff (2001). "ANL human health fact sheet--plutonium". Argonne National Laboratory. http://consolidationeis.doe.gov/PDFs/PlutoniumANLFactSheetOct2001.pdf. Retrieved 2007-06-16.
- ↑ Cohen, Bernard L.. "The Nuclear Energy Option, Chapter 13, Plutonium and Bombs". http://www.phyast.pitt.edu/~blc/book/chapter13.html.
- ↑ Voelz, G. L. (1975). "What We Have Learned About Plutonium from Human Data". The Radiation Safety Journal Health Physics: 29. http://journals.lww.com/health-physics/Abstract/1975/10000/What_We_Have_Learned_about_Plutonium_from_Human.11.aspx.
- ↑ Welsome, Eileen (2000). The Plutonium Files: America's Secret Medical Experiments in the Cold War. New York (NY): Random House. p. 17. ISBN 0-385-31954-1.
- ↑ Miner 1968, p. 546
- ↑ Roark, Kevin N. (2000). Criticality accidents report issued. Los Alamos (NM): Los Alamos National Laboratory. http://www.lanl.gov/news/index.php/fuseaction/home.story/story_id/1054/view/print. Retrieved 2008-11-16.
- ↑ LANL contributors. "Raemer Schreiber". Staff Biographies. Los Alamos (NM): Los Alamos National Laboratory. http://www.lanl.gov/history/people/R_Schreiber.shtml. Retrieved 2008-11-16.
- ↑ 95.0 95.1 McLaughlin, Thomas P.; Monahan, Shean P.; Pruvost, Norman L. (2000). A Review of Criticality Accidents. Los Alamos (NM): Los Alamos National Laboratory. p. 16. LA-13638.
- ↑ 96.0 96.1 DOE contributors. "Plutonium". Nuclear Safety and the Environment. Department of Energy, Office of Health Safety and Security. http://www.hss.energy.gov/nuclearsafety/ns/techstds/standard/hdbk1081/hbk1081d.html#ZZ281. Retrieved 2008-12-07.
- ↑ DOE contributors (1994). "Primer on Spontaneous Heating and Pyrophoricity – Pyrophoric Metals – Plutonium". Washington (DC): U.S. Department of Energy, Office of Nuclear Safety, Quality Assurance and Environment. Archived from the original on 2007-04-28. http://web.archive.org/web/20070428220410/http://www.hss.energy.gov/NuclearSafety/techstds/standard/hdbk1081/hbk1081d.html#ZZ281.
Bibliography
- CRC contributors (2006). David R. Lide. ed. Handbook of Chemistry and Physics (87th ed.). Boca Raton (FL): CRC Press, Taylor & Francis Group. ISBN 0849304873.
- Emsley, John (2001). "Plutonium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford (UK): Oxford University Press. pp. 324–329. ISBN 0198503407.
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford (UK): Butterworth-Heinemann. ISBN 0-7506-3365-4.
- Heiserman, David L. (1992). "Element 94: Plutonium". Exploring Chemical Elements and their Compounds. New York (NY): TAB Books. pp. 337–340. ISBN 0-8306-3018-X.
- Miner, William N.; Schonfeld, Fred W. (1968). "Plutonium". In Clifford A. Hampel (editor). The Encyclopedia of the Chemical Elements. New York (NY): Reinhold Book Corporation. pp. 540–546. LCCN 68-29938.
- Stwertka, Albert (1998). "Plutonium". Guide to the Elements (Revised ed.). Oxford (UK): Oxford University Press. ISBN 0-19-508083-1.
External links
- Sutcliffe, W.G.; et al. (1995). "A Perspective on the Dangers of Plutonium". Lawrence Livermore National Laboratory. Archived from the original on 2006-09-29. http://web.archive.org/web/20060929015050/http://www.llnl.gov/csts/publications/sutcliffe/.
- Johnson, C.M.; Davis, Z.S. (1997). "Nuclear Weapons: Disposal Options for Surplus Weapons-Usable Plutonium". CRS Report for Congress # 97-564 ENR. http://www.globalsecurity.org/wmd/library/report/crs/97-564.htm. Retrieved 2009-02-15.
- IEER contributors (2005). "Physical, Nuclear, and Chemical, Properties of Plutonium". IEER. http://www.ieer.org/fctsheet/pu-props.html. Retrieved 2009-02-15.
- Bhadeshia, H.. "Plutonium crystallography". http://www.msm.cam.ac.uk/phase-trans/2006/Plutonium/Plutonium.html.
- Samuels, D. (2005). "End of the Plutonium Age". Discover Magazine 26 (11). http://discovermagazine.com/2005/nov/end-of-plutonium.
- Pike, J.; Sherman, R. (2000). "Plutonium production". Federation of American Scientists. http://www.fas.org/nuke/intro/nuke/plutonium.htm. Retrieved 2009-02-15.
- Nuclear Weapon Archive contributors. "Plutonium Manufacture and Fabrication". Nuclearweaponarchive.org. http://nuclearweaponarchive.org/Library/Plutonium/.
- Ong, C. (1999). "World Plutonium Inventories". Nuclear Files.org. http://www.nuclearfiles.org/menu/key-issues/nuclear-energy/issues/world-plutonium-inventories-ong.htm. Retrieved 2009-02-15.
- LANL contributors (2000). "Challenges in Plutonium Science". Los Alamos Science I & II (26). http://www.fas.org/sgp/othergov/doe/lanl/pubs/number26.htm. Retrieved 2009-02-15.
- NLM contributors. "Plutonium, Radioactive". NLM Hazardous Substances Databank. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@na+@rel+plutonium,+radioactive. Retrieved 2009-02-15.
- Alsos contributors. "Annotated Bibliography on plutonium". Alsos Digital Library for Nuclear Issues. http://alsos.wlu.edu/qsearch.aspx?browse=science/Plutonium. Retrieved 2009-02-15.
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Plutonium
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
![\mathrm{^{238}_{\ 92}U\ +\ ^{1}_{0}n\ \longrightarrow \ ^{239}_{\ 92}U\ \xrightarrow[23.5 \ min]{\beta^-} \ ^{239}_{\ 93}Np\ \xrightarrow[2.3565 \ d]{\beta^-} \ ^{239}_{\ 94}Pu}](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/dceb3c6a1ace80ddc1a9f556ac671d77.png)
![\mathrm{^{238}_{\ 92}U\ +\ ^{2}_{1}D\ \longrightarrow \ ^{238}_{\ 93}Np\ +\ 2\ ^{1}_{0}n \quad;\quad ^{238}_{\ 93}Np\ \xrightarrow[2.117 \ d]{\beta^-} \ ^{238}_{\ 94}Pu}](/2010-wikipedia_en_wp1-0.8_orig_2010-12/I/af20566ad2dd7bb3effcde39b53f3e47.png)
