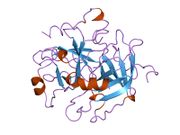
Thrombin
| Coagulation factor II (thrombin) |

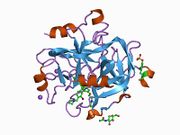
The structure of human thrombin in complex with the inhibitor hirudin.[1] |
| Available structures |
| PDB |
1a2c, 1a3b, 1a3e, 1a46, 1a4w, 1a5g, 1a61, 1abi, 1abj, 1ad8, 1ae8, 1afe, 1aht, 1ai8, 1aix, 1awf, 1awh, 1ay6, 1b5g, 1b7x, 1ba8, 1bb0, 1bbr, 1bcu, 1bhx, 1bmm, 1bmn, 1bth, 1c1u, 1c1v, 1c1w, 1c4u, 1c4v, 1c4y, 1c5l, 1c5n, 1c5o, 1ca8, 1d3d, 1d3p, 1d3q, 1d3t, 1d4p, 1d6w, 1d9i, 1de7, 1dit, 1dm4, 1doj, 1dwb, 1dwc, 1dwd, 1dwe, 1dx5, 1e0f, 1eb1, 1eoj, 1eol, 1fpc, 1fph, 1g30, 1g32, 1g37, 1ghv, 1ghw, 1ghx, 1ghy, 1gj4, 1gj5, 1h8d, 1h8i, 1hag, 1hah, 1hai, 1hao, 1hap, 1hbt, 1hdt, 1hgt, 1hlt, 1hut, 1hxe, 1hxf, 1ihs, 1iht, 1jmo, 1jou, 1jwt, 1k21, 1k22, 1kts, 1ktt, 1lhc, 1lhd, 1lhe, 1lhf, 1lhg, 1mh0, 1mu6, 1mu8, 1mue, 1nm6, 1no9, 1nrn, 1nro, 1nrp, 1nrq, 1nrr, 1nrs, 1nt1, 1nu7, 1nu9, 1ny2, 1nzq, 1o0d, 1o2g, 1o5g, 1ook, 1oyt, 1p8v, 1ppb, 1qbv, 1qhr, 1qj1, 1qj6, 1qj7, 1qur, 1rd3, 1riw, 1sb1, 1sfq, 1sg8, 1sgi, 1shh, 1sl3, 1sr5, 1t4u, 1t4v, 1ta2, 1ta6, 1tb6, 1tbz, 1thp, 1thr, 1ths, 1tmb, 1tmt, 1tmu, 1tom, 1tq0, 1tq7, 1twx, 1ucy, 1uma, 1uvs, 1vit, 1vr1, 1vzq, 1w7g, 1way, 1wbg, 1xm1, 1xmn, 1ycp, 1ype, 1ypg, 1ypj, 1ypk, 1ypl, 1ypm, 1z71, 1z8i, 1z8j, 1zgi, 1zgv, 1zrb, 2a0q, 2a2x, 2a45, 2afq, 2ank, 2anm, 2b5t, 2bdy, 2bvr, 2bvs, 2bvx, 2bxt, 2bxu, 2c8w, 2c8x, 2c8y, 2c8z, 2c90, 2c93, 2cf8, 2cf9, 2cn0, 2feq, 2fes, 2gde, 2gp9, 2h9t, 2hgt, 2hnt, 2hpp, 2hpq, 2hwl, 2jh0, 2jh5, 2jh6, 2od3, 2thf, 3hat, 3htc, 4htc, 4thn, 5gds, 7kme, 8kme |
|
| Identifiers |
| Symbols |
F2; PT |
| External IDs |
OMIM: 176930 MGI: 88380 HomoloGene: 426 GeneCards: F2 Gene |
| EC number |
3.4.21.5 |
|
|
| RNA expression pattern |
 |
| More reference expression data |
| Orthologs |
| Species |
Human |
Mouse |
|
| Entrez |
2147 |
14061 |
|
| Ensembl |
ENSG00000180210 |
ENSMUSG00000027249 |
|
| UniProt |
P00734 |
Q3TJ94 |
|
| RefSeq (mRNA) |
NM_000506 |
NM_010168 |
|
| RefSeq (protein) |
NP_000497 |
NP_034298 |
|
| Location (UCSC) |
Chr 11:
46.7 - 46.72 Mb |
Chr 2:
91.43 - 91.44 Mb |
|
| PubMed search |
[1] |
[2] |
|
Thrombin (activated Factor II [IIa]) is a coagulation protein in the blood stream that has many effects in the coagulation cascade. It is a serine protease (EC 3.4.21.5) that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.
Genetics
The Thrombin (prothrombin) gene is located on the eleventh chromosome (11p11-q12). The molecular weight of prothrombin is approximately 72000 gmol-1. The catalytic domain is released from prothrombin fragment 1.2 to create the active enzyme thrombin, which has a molecular weight of 36000 gmol-1.
There are an estimated 30 people in the world that have been diagnosed with the congenital form of Factor II deficiency,[2] which should not be confused with a mutation of prothrombin. The prothrombin gene mutation is called Factor II mutation. Factor II mutation is congenital.[3] The Factor II mutated gene is not usually accompanied by other factor mutations (i.e. the most common is Factor V Leiden). The gene may be inherited heterozygous (1 pair), or much more rarely, homozygous (2 pairs), and is not related to gender or blood type. Homozygous mutations increase the risk of thrombosis more than heterozygous mutations, but the relative increased risk is not well documented. Other potential risks for thrombosis, such as oral contraceptives may be additive. The previously reported relationship of inflammatory bowel disease (i.e. Crohn's disease or Ulcerative Colitis) and prothrombin mutation or Factor V Leiden mutation have been contradicted by research.[4]

Anchoring of bovine prothrombin to the membrane through its Gla domain
Physiology
Generation
Thrombin is produced by the enzymatic cleavage of two sites on prothrombin by activated Factor X (Xa). The activity of factor Xa is greatly enhanced by binding to activated Factor V (Va), termed the prothrombinase complex. Prothrombin is produced in the liver and is post-translationally modified in a vitamin K-dependent reaction that converts ten glutamic acids on prothrombin to gamma-carboxyglutamic acid (Gla). In the presence of calcium, the Gla residues promote the binding of prothrombin to phospholipid bilayers (see the picture). Deficiency of vitamin K or administration of the anticoagulant warfarin inhibits the production of gamma-carboxyglutamic acid residues, slowing the activation of the coagulation cascade.
In human adults the normal blood level of antithrombin activity has been measured to be around 1.1 units /ml. Newborn levels of thrombin steadily increase after birth to reach normal adult levels, from a level of around 0.5 units/ml 1 day after birth, to a level of around 0.9 units/ml after 6 months of life.[5]
Action
Thrombin converts fibrinogen to an active form that assembles into fibrin. Thrombin also activates factor XI,[6] factor V, and factor VIII. This positive feedback accelerates the production of thrombin.
Factor XIII is also activated by thrombin. Factor XIIIa is a transglutaminase that catalyzes the formation of covalent bonds between lysine and glutamine residues in fibrin. The covalent bonds increase the stability of the fibrin clot.
Platelets
In addition to its activity in the coagulation cascades, thrombin also promotes platelet activation, via activation of protease-activated receptors on the platelet.
Negative feedback
Thrombin bound to thrombomodulin activates protein C, an inhibitor of the coagulation cascade. The activation of protein C is greatly enhanced following the binding of thrombin to thrombomodulin, an integral membrane protein expressed by endothelial cells. Activated protein C inactivates factors Va and VIIIa. Binding of activated protein C to protein S leads to a modest increase in its activity.
Role in disease
Activation of prothrombin is crucial in physiological and pathological coagulation. Various rare diseases involving prothrombin have been described (e.g., hypoprothrombinemia). Anti-thrombin antibodies in autoimmune disease may be a factor in the formation of the lupus anticoagulant also known as (antiphospholipid syndrome). Hyperprothrombinemia can be caused by a mutation at 20210a.
Thrombin, a potent vasoconstrictor and mitogen, is implicated as a major factor in vasospasm following subarachnoid hemorrhage. Blood from a ruptured cerebral aneurysm clots around a cerebral artery, releasing thrombin. This can induce an acute and prolonged narrowing of the blood vessel, potentially resulting in cerebral ischemia and infarction (stroke).
Biotechnology
Due to its high proteolytic specificity, thrombin is a valuable biochemical tool. The thrombin cleavage site (Leu-Val-Pro-Arg-Gly-Ser) is commonly included in linker regions of recombinant fusion protein constructs. Following purification of the fusion protein, thrombin can be used to selectively cleave between the Arginine and Glycine residues of the cleavage site, effectively removing the purification tag from the protein of interest with a high degree of specificity.
Pharmacology
Prothrombin complex concentrate and fresh frozen plasma are prothrombin-rich coagulation factor preparations that can be used to correct deficiencies (usually due to medication) of prothrombin. Indications include intractable bleeding due to warfarin.
Manipulation of prothrombin is central to the mode of action of most anticoagulants. Warfarin and related drugs inhibit vitamin K-dependent carboxylation of several coagulation factors, including prothrombin. Heparin increases the affinity of antithrombin to thrombin (as well as factor Xa). The direct thrombin inhibitors, a newer class of medication, directly inhibit thrombin by binding to its active site.
Use in food production
Thrombin is sold under the brand name Fibrimex for use as a binding agent for meat. The thrombin in Fibrimex derives from pigs or bovine blood.[7] According to the manufacturer it can be used to produce new kinds of mixed meats (for example combining beef and fish seamlessly). The manufacturer also states that it can be used to combine scrap meat into whole steaks which can then be sold to a higher price, thus cutting down on production costs.[8]
Criticism against use in food production
General secretary Jan Bertoft of Sveriges Konsumenter (The Consumer Coalition of Sweden) has stated that "there is danger of misleading the consumers since there is no way to tell this reconstituted meat from real meat"[7]
History
After the description of fibrinogen and fibrin, Alexander Schmidt hypothesised the existence of an enzyme that converts fibrinogen into fibrin in 1872.[9]
Interactions
Thrombin has been shown to interact with Thrombomodulin.[10][11]
See also
- The Proteolysis Map
- Fibrin glue
External links
References
- ↑ PDB 2C93; Howard N, Abell C, Blakemore W, Chessari G, Congreve M, Howard S, Jhoti H, Murray CW, Seavers LC, van Montfort RL (February 2006). "Application of fragment screening and fragment linking to the discovery of novel thrombin inhibitors". J. Med. Chem. 49 (4): 1346–55. doi:10.1021/jm050850v. PMID 16480269.
- ↑ Degen SJ, McDowell SA, et al. (1995). "Prothrombin Frankfurt: a dysfunctional prothrombin characterized by substitution of Glu-466 by Ala". Thromb. Haemost. 73 (2): 203–209. PMID 7792730.
- ↑ Varga EA, Moll S. (2004). "Cardiology patient pages. Prothrombin 20210 mutation (factor II mutation)". Circulation 110 (3): e15–8. doi:10.1161/01.CIR.0000135582.53444.87. PMID 15262854.
- ↑ Bernstein CN, Sargent M, et al. (2007). "Mutations in clotting factors and inflammatory bowel disease". Am. J. Gastroenterol. 102 (2): 338–343. doi:10.1111/j.1572-0241.2006.00974.x. PMID 17156138.
- ↑ Andrew M, Paes B et al. (1987). "Development of the human coagulation system in the full-term infant". Blood 70 (1): 165–172. PMID 3593964.
- ↑ Oliver J, Monroe D et al. (1999). "Thrombin Activates Factor XI on Activated Platelets in the Absence of Factor XII". Arteriosclerosis, Thrombosis, and Vascular Biology 19: 170–177. http://atvb.ahajournals.org/cgi/reprint/19/1/170.pdf.
- ↑ 7.0 7.1 "Sverige röstade ja till köttklister", Dagens nyheter, Stockholm, February 9, 2010.
- ↑ Fibrimex website
- ↑ Schmidt A (1872). "Neue Untersuchungen ueber die Fasserstoffesgerinnung". Pflüger's Archiv für die gesamte Physiologie 6: 413–538. doi:10.1007/BF01612263.
- ↑ Bajzar, L; Morser J, Nesheim M (Jul. 1996). "TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex". J. Biol. Chem. (UNITED STATES) 271 (28): 16603–8. doi:10.1074/jbc.271.28.16603. ISSN 0021-9258. PMID 8663147.
- ↑ Jakubowski, H V; Owen W G (Jul. 1989). "Macromolecular specificity determinants on thrombin for fibrinogen and thrombomodulin". J. Biol. Chem. (UNITED STATES) 264 (19): 11117–21. ISSN 0021-9258. PMID 2544585.
Further reading
- Esmon CT (1995). "Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface". FASEB J 9 (10): 946–55. PMID 7615164.
- Lenting PJ, van Mourik JA, Mertens K (1999). "The life cycle of coagulation factor VIII in view of its structure and function". Blood 92 (11): 3983–96. PMID 9834200.
- Plow EF, Cierniewski CS, Xiao Z, et al. (2002). "AlphaIIbbeta3 and its antagonism at the new millennium". Thromb. Haemost 86 (1): 34–40. PMID 11487023.
- Maragoudakis ME, Tsopanoglou NE, Andriopoulou P (2002). "Mechanism of thrombin-induced angiogenesis". Biochem. Soc. Trans 30 (2): 173–7. doi:10.1042/ (inactive 2008-06-28). PMID 12023846.
- Howell DC, Laurent GJ, Chambers RC (2002). "Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis". Biochem. Soc. Trans 30 (2): 211–6. doi:10.1042/ (inactive 2008-06-28). PMID 12023853.
- Firth SM, Baxter RC (2003). "Cellular actions of the insulin-like growth factor binding proteins". Endocr. Rev 23 (6): 824–54. doi:10.1210/er.2001-0033. PMID 12466191.
- Minami T, Sugiyama A, Wu SQ, et al. (2004). "Thrombin and phenotypic modulation of the endothelium". Arterioscler. Thromb. Vasc. Biol 24 (1): 41–53. doi:10.1161/01.ATV.0000099880.09014.7D. PMID 14551154.
- De Cristofaro R, De Candia E (2004). "Thrombin domains: structure, function and interaction with platelet receptors". J. Thromb. Thrombolysis 15 (3): 151–63. doi:10.1023/B:THRO.0000011370.80989.7b. PMID 14739624.
- Tsopanoglou NE, Maragoudakis ME (2004). "Role of thrombin in angiogenesis and tumor progression". Semin. Thromb. Hemost 30 (1): 63–9. doi:10.1055/s-2004-822971. PMID 15034798.
- Bode W (2007). "Structure and interaction modes of thrombin". Blood Cells Mol. Dis 36 (2): 122–30. doi:10.1016/j.bcmd.2005.12.027. PMID 16480903.
- Wolberg AS (2007). "Thrombin generation and fibrin clot structure". Blood Rev 21 (3): 131–42. doi:10.1016/j.blre.2006.11.001. PMID 17208341.
- Degen S: Prothrombin. In: High K, Roberts H, eds. Molecular Basis of Thrombosis and Hemostasis. New York, NY: Marcel Dekker; 1995:75.
|
PDB Gallery |
|
|
|
|
1a2c: STRUCTURE OF THROMBIN INHIBITED BY AERUGINOSIN298-A FROM A BLUE-GREEN ALGA
|
|
|
|
1a3b: COMPLEX OF HUMAN ALPHA-THROMBIN WITH THE BIFUNCTIONAL BORONATE INHIBITOR BOROLOG1
|
|
|
|
1a3e: COMPLEX OF HUMAN ALPHA-THROMBIN WITH THE BIFUNCTIONAL BORONATE INHIBITOR BOROLOG2
|
|
|
|
1a46: THROMBIN COMPLEXED WITH HIRUGEN AND A BETA-STRAND MIMETIC INHIBITOR
|
|
|
|
1a4w: CRYSTAL STRUCTURES OF THROMBIN WITH THIAZOLE-CONTAINING INHIBITORS: PROBES OF THE S1' BINDING SITE
|
|
|
|
1a5g: HUMAN THROMBIN COMPLEXED WITH NOVEL SYNTHETIC PEPTIDE MIMETIC INHIBITOR AND HIRUGEN
|
|
|
|
1a61: THROMBIN COMPLEXED WITH A BETA-MIMETIC THIAZOLE-CONTAINING INHIBITOR
|
|
|
|
1abi: STRUCTURE OF THE HIRULOG 3-THROMBIN COMPLEX AND NATURE OF THE S' SUBSITES OF SUBSTRATES AND INHIBITORS
|
|
|
|
1abj: STRUCTURE OF THE HIRULOG 3-THROMBIN COMPLEX AND NATURE OF THE S' SUBSITES OF SUBSTRATES AND INHIBITORS
|
|
|
|
1ad8: COMPLEX OF THROMBIN WITH AND INHIBITOR CONTAINING A NOVEL P1 MOIETY
|
|
|
|
1ae8: HUMAN ALPHA-THROMBIN INHIBITION BY EOC-D-PHE-PRO-AZALYS-ONP
|
|
|
|
1aht: CRYSTAL STRUCTURE OF HUMAN ALPHA-THROMBIN COMPLEXED WITH HIRUGEN AND P-AMIDINOPHENYLPYRUVATE) AT 1.6 ANGSTROMS RESOLUTION
|
|
|
|
1ai8: HUMAN ALPHA-THROMBIN TERNARY COMPLEX WITH THE EXOSITE INHIBITOR HIRUGEN AND ACTIVE SITE INHIBITOR PHCH2OCO-D-DPA-PRO-BOROMPG
|
|
|
|
1aix: HUMAN ALPHA-THROMBIN TERNARY COMPLEX WITH EXOSITE INHIBITOR HIRUGEN AND ACTIVE SITE INHIBITOR PHCH2OCO-D-DPA-PRO-BOROVAL
|
|
|
|
1awf: NOVEL COVALENT THROMBIN INHIBITOR FROM PLANT EXTRACT
|
|
|
|
1awh: NOVEL COVALENT THROMBIN INHIBITOR FROM PLANT EXTRACT
|
|
|
|
1ay6: THROMBIN INHIBITOR FROM THEONALLA, CYCLOTHEANAMIDE-BASED MACROCYCLIC TRIPEPTIDE MOTIF
|
|
|
|
1b5g: HUMAN THROMBIN COMPLEXED WITH NOVEL SYNTHETIC PEPTIDE MIMETIC INHIBITOR AND HIRUGEN
|
|
|
|
1b7x: STRUCTURE OF HUMAN ALPHA-THROMBIN Y225I MUTANT BOUND TO D-PHE-PRO-ARG-CHLOROMETHYLKETONE
|
|
|
|
1ba8: THROMBIN INHIBITOR WITH A RIGID TRIPEPTIDYL ALDEHYDES
|
|
|
|
1bb0: THROMBIN INHIBITORS WITH RIGID TRIPEPTIDYL ALDEHYDES
|
|
|
|
1bbr: THE STRUCTURE OF RESIDUES 7-16 OF THE A ALPHA CHAIN OF HUMAN FIBRINOGEN BOUND TO BOVINE THROMBIN AT 2.3 ANGSTROMS RESOLUTION
|
|
|
|
1bcu: ALPHA-THROMBIN COMPLEXED WITH HIRUGEN AND PROFLAVIN
|
|
|
|
1bhx: X-RAY STRUCTURE OF THE COMPLEX OF HUMAN ALPHA THROMBIN WITH THE INHIBITOR SDZ 229-357
|
|
|
|
1bth: STRUCTURE OF THROMBIN COMPLEXED WITH BOVINE PANCREATIC TRYPSIN INHIBITOR
|
|
|
|
1c1u: RECRUITING ZINC TO MEDIATE POTENT, SPECIFIC INHIBITION OF SERINE PROTEASES
|
|
|
|
1c1v: RECRUITING ZINC TO MEDIATE POTENT, SPECIFIC INHIBITION OF SERINE PROTEASES
|
|
|
|
1c1w: RECRUITING ZINC TO MEDIATE POTENT, SPECIFIC INHIBITION OF SERINE PROTEASES
|
|
|
|
1c4u: SELECTIVE NON ELECTROPHILIC THROMBIN INHIBITORS WITH CYCLOHEXYL MOIETIES.
|
|
|
|
1c4v: SELECTIVE NON ELECTROPHILIC THROMBIN INHIBITORS WITH CYCLOHEXYL MOIETIES.
|
|
|
|
1c4y: SELECTIVE NON-ELECTROPHILIC THROMBIN INHIBITORS
|
|
|
|
1c5l: STRUCTURAL BASIS FOR SELECTIVITY OF A SMALL MOLECULE, S1-BINDING, SUB-MICROMOLAR INHIBITOR OF UROKINASE TYPE PLASMINOGEN ACTIVATOR
|
|
|
|
1c5n: STRUCTURAL BASIS FOR SELECTIVITY OF A SMALL MOLECULE, S1-BINDING, SUB-MICROMOLAR INHIBITOR OF UROKINASE TYPE PLASMINOGEN ACTIVATOR
|
|
|
|
1c5o: STRUCTURAL BASIS FOR SELECTIVITY OF A SMALL MOLECULE, S1-BINDING, SUB-MICROMOLAR INHIBITOR OF UROKINASE TYPE PLASMINOGEN ACTIVATOR
|
|
|
|
1ca8: THROMBIN INHIBITORS WITH RIGID TRIPEPTIDYL ALDEHYDES
|
|
|
|
1d3d: CRYSTAL STRUCTURE OF HUMAN ALPHA THROMBIN IN COMPLEX WITH BENZOTHIOPHENE INHIBITOR 4
|
|
|
|
1d3p: CRYSTAL STRUCTURE OF HUMAN APLHA-THROMBIN IN COMPLEX WITH BENZO[B]THIOPHENE INHIBITOR 3
|
|
|
|
1d3q: CRYSTAL STRUCTURE OF HUMAN ALPHA THROMBIN IN COMPLEX WITH BENZO[B]THIOPHENE INHIBITOR 2
|
|
|
|
1d3t: CRYSTAL STRUCTURE OF HUMAN ALPHA THROMBIN IN COMPLEX WITH BENZO[B]THIOPHENE INHIBITOR 1
|
|
|
|
1d4p: CRYSTAL STRUCTURE OF HUMAN ALPHA THROMBIN IN COMPLEX WITH 5-AMIDINOINDOLE-4-BENZYLPIPERIDINE INHIBITOR
|
|
|
|
1d6w: STRUCTURE OF THROMBIN COMPLEXED WITH SELECTIVE NON-ELECTROPHILIC INHIBITORS HAVING CYCLOHEXYL MOIETIES AT P1
|
|
|
|
1d9i: STRUCTURE OF THROMBIN COMPLEXED WITH SELECTIVE NON-ELECTOPHILIC INHIBITORS HAVING CYCLOHEXYL MOIETIES AT P1
|
|
|
|
1de7: INTERACTION OF FACTOR XIII ACTIVATION PEPTIDE WITH ALPHA-THROMBIN: CRYSTAL STRUCTURE OF THE ENZYME-SUBSTRATE COMPLEX
|
|
|
|
1dit: COMPLEX OF A DIVALENT INHIBITOR WITH THROMBIN
|
|
|
|
1dm4: SER195ALA MUTANT OF HUMAN THROMBIN COMPLEXED WITH FIBRINOPEPTIDE A (7-16)
|
|
|
|
1doj: Crystal structure of human alpha-thrombin*RWJ-51438 complex at 1.7 A
|
|
|
|
1dwb: CRYSTALLOGRAPHIC ANALYSIS AT 3.0-ANGSTROMS RESOLUTION OF THE BINDING TO HUMAN THROMBIN OF FOUR ACTIVE SITE-DIRECTED INHIBITORS
|
|
|
|
1dwc: CRYSTALLOGRAPHIC ANALYSIS AT 3.0-ANGSTROMS RESOLUTION OF THE BINDING TO HUMAN THROMBIN OF FOUR ACTIVE SITE-DIRECTED INHIBITORS
|
|
|
|
1dwd: CRYSTALLOGRAPHIC ANALYSIS AT 3.0-ANGSTROMS RESOLUTION OF THE BINDING TO HUMAN THROMBIN OF FOUR ACTIVE SITE-DIRECTED INHIBITORS
|
|
|
|
1dwe: CRYSTALLOGRAPHIC ANALYSIS AT 3.0-ANGSTROMS RESOLUTION OF THE BINDING TO HUMAN THROMBIN OF FOUR ACTIVE SITE-DIRECTED INHIBITORS
|
|
|
|
1dx5: CRYSTAL STRUCTURE OF THE THROMBIN-THROMBOMODULIN COMPLEX
|
|
|
|
1e0f: CRYSTAL STRUCTURE OF THE HUMAN ALPHA-THROMBIN-HAEMADIN COMPLEX: AN EXOSITE II-BINDING INHIBITOR
|
|
|
|
1eb1: COMPLEX STRUCTURE OF HUMAN THROMBIN WITH N-METHYL-ARGININE INHIBITOR
|
|
|
|
1eoj: DESIGN OF P1' AND P3' RESIDUES OF TRIVALENT THROMBIN INHIBITORS AND THEIR CRYSTAL STRUCTURES
|
|
|
|
1eol: DESIGN OF P1' AND P3' RESIDUES OF TRIVALENT THROMBIN INHIBITORS AND THEIR CRYSTAL STRUCTURES
|
|
|
|
1fpc: ACTIVE SITE MIMETIC INHIBITION OF THROMBIN
|
|
|
|
1fph: THE INTERACTION OF THROMBIN WITH FIBRINOGEN: A STRUCTURAL BASIS FOR ITS SPECIFICITY
|
|
|
|
1g30: THROMBIN INHIBITOR COMPLEX
|
|
|
|
1g32: THROMBIN INHIBITOR COMPLEX
|
|
|
|
1g37: CRYSTAL STRUCTURE OF HUMAN ALPHA-THROMBIN COMPLEXED WITH BCH-10556 AND EXOSITE-DIRECTED PEPTIDE
|
|
|
|
1ghv: A NOVEL SERINE PROTEASE INHIBITION MOTIF INVOLVING A MULTI-CENTERED SHORT HYDROGEN BONDING NETWORK AT THE ACTIVE SITE
|
|
|
|
1ghw: A NOVEL SERINE PROTEASE INHIBITION MOTIF INVOLVING A MULTI-CENTERED SHORT HYDROGEN BONDING NETWORK AT THE ACTIVE SITE
|
|
|
|
1ghx: A NOVEL SERINE PROTEASE INHIBITION MOTIF INVOLVING A MULTI-CENTERED SHORT HYDROGEN BONDING NETWORK AT THE ACTIVE SITE
|
|
|
|
1ghy: A NOVEL SERINE PROTEASE INHIBITION MOTIF INVOLVING A MULTI-CENTERED SHORT HYDROGEN BONDING NETWORK AT THE ACTIVE SITE
|
|
|
|
1gj4: SELECTIVITY AT S1, H2O DISPLACEMENT, UPA, TPA, SER190/ALA190 PROTEASE, STRUCTURE-BASED DRUG DESIGN
|
|
|
|
1gj5: SELECTIVITY AT S1, H2O DISPLACEMENT, UPA, TPA, SER190/ALA190 PROTEASE, STRUCTURE-BASED DRUG DESIGN
|
|
|
|
1h8d: X-RAY STRUCTURE OF THE HUMAN ALPHA-THROMBIN COMPLEX WITH A TRIPEPTIDE PHOSPHONATE INHIBITOR.
|
|
|
|
1h8i: X-RAY CRYSTAL STRUCTURE OF HUMAN ALPHA-THROMBIN WITH A TRIPEPTIDE PHOSPHONATE INHIBITOR.
|
|
|
|
1hag: THE ISOMORPHOUS STRUCTURES OF PRETHROMBIN2, HIRUGEN-AND PPACK-THROMBIN: CHANGES ACCOMPANYING ACTIVATION AND EXOSITE BINDING TO THROMBIN
|
|
|
|
1hah: THE ISOMORPHOUS STRUCTURES OF PRETHROMBIN2, HIRUGEN-AND PPACK-THROMBIN: CHANGES ACCOMPANYING ACTIVATION AND EXOSITE BINDING TO THROMBIN
|
|
|
|
1hai: THE ISOMORPHOUS STRUCTURES OF PRETHROMBIN2, HIRUGEN-AND PPACK-THROMBIN: CHANGES ACCOMPANYING ACTIVATION AND EXOSITE BINDING TO THROMBIN
|
|
|
|
1hao: COMPLEX OF HUMAN ALPHA-THROMBIN WITH A 15MER OLIGONUCLEOTIDE GGTTGGTGTGGTTGG (BASED ON NMR MODEL OF DNA
|
|
|
|
1hap: COMPLEX OF HUMAN ALPHA-THROMBIN WITH A 15MER OLIGONUCLEOTIDE GGTTGGTGTGGTTGG (BASED ON X-RAY MODEL OF DNA)
|
|
|
|
1hbt: HUMAN ALPHA-THROMBIN COMPLEXED WITH A PEPTIDYL PYRIDINIUM METHYL KETONE CONTAINING BIVALENT INHIBITOR
|
|
|
|
1hdt: STRUCTURE OF A RETRO-BINDING PEPTIDE INHIBITOR COMPLEXED WITH HUMAN ALPHA-THROMBIN
|
|
|
|
1hgt: STRUCTURE OF THE HIRUGEN AND HIRULOG 1 COMPLEXES OF ALPHA-THROMBIN
|
|
|
|
1hlt: THE STRUCTURE OF A NONADECAPEPTIDE OF THE FIFTH EGF DOMAIN OF THROMBOMODULIN COMPLEXED WITH THROMBIN
|
|
|
|
1hut: THE STRUCTURE OF ALPHA-THROMBIN INHIBITED BY A 15-MER SINGLE-STRANDED DNA APTAMER
|
|
|
|
1hxf: HUMAN THROMBIN COMPLEX WITH HIRUDIN VARIANT
|
|
|
|
1ihs: CRYSTAL STRUCTURE OF THE COMPLEX OF HUMAN ALPHA-THROMBIN AND NON-HYDROLYZABLE BIFUNCTIONAL INHIBITORS, HIRUTONIN-2 AND HIRUTONIN-6
|
|
|
|
1iht: CRYSTAL STRUCTURE OF THE COMPLEX OF HUMAN ALPHA-THROMBIN AND NON-HYDROLYZABLE BIFUNCTIONAL INHIBITORS, HIRUTONIN-2 AND HIRUTONIN-6
|
|
|
|
1jmo: Crystal Structure of the Heparin Cofactor II-S195A Thrombin Complex
|
|
|
|
1jou: Crystal Structure of Native S195A Thrombin with an Unoccupied Active Site
|
|
|
|
1jwt: CRYSTAL STRUCTURE OF THROMBIN IN COMPLEX WITH A NOVEL BICYCLIC LACTAM INHIBITOR
|
|
|
|
1k21: HUMAN THROMBIN-INHIBITOR COMPLEX
|
|
|
|
1k22: HUMAN THROMBIN-INHIBITOR COMPLEX
|
|
|
|
1kts: Thrombin Inhibitor Complex
|
|
|
|
1ktt: Thrombin inhibitor complex
|
|
|
|
1lhc: HUMAN ALPHA-THROMBIN COMPLEXED WITH AC-(D)PHE-PRO-BOROARG-OH
|
|
|
|
1lhd: HUMAN ALPHA-THROMBIN COMPLEXED WITH AC-(D)PHE-PRO-BOROLYS-OH
|
|
|
|
1lhe: HUMAN ALPHA-THROMBIN COMPLEXED WITH AC-(D)PHE-PRO-BORO-N-BUTYL-AMIDINO-GLYCINE-OH
|
|
|
|
1lhf: HUMAN ALPHA-THROMBIN COMPLEXED WITH AC-(D)PHE-PRO-BORO-HOMOLYS-OH
|
|
|
|
1lhg: HUMAN ALPHA-THROMBIN COMPLEXED WITH AC-(D)PHE-PRO-BOROORNITHINE-OH
|
|
|
|
1mh0: Crystal structure of the anticoagulant slow form of thrombin
|
|
|
|
1mu6: Crystal Structure of Thrombin in Complex with L-378,622
|
|
|
|
1mu8: thrombin-hirugen_l-378,650
|
|
|
|
1mue: Thrombin-Hirugen-L405,426
|
|
|
|
1nm6: thrombin in complex with selective macrocyclic inhibitor at 1.8A
|
|
|
|
1no9: Design of weakly basic thrombin inhibitors incorporating novel P1 binding functions: molecular and X-ray crystallographic studies.
|
|
| The maximum number of images (100) is exceeded ! |
|
|
|
Proteins: coagulation |
|
| Coagulation factors |
|
|
vWF
platelet membrane glycoproteins: Ib (A, B, IX) · IIb/IIIa (IIb, IIIa) · VI
|
|
|
|
HMWK/Bradykinin · Prekallikrein/Kallikrein · XII "Hageman"
XI · IX · VIII
|
|
|
|
III "Tissue factor" · VII
|
|
|
|
|
|
|
| Coagulation inhibitors |
Antithrombin (inhibits II, IX, X, XI, XII) · Protein C (inhibits V, VIII)/Protein S (cofactor for protein C) · Protein Z (inhibits X) · ZPI (inhibits X, XI) · TFPI (inhibits III)
|
|
| Thrombolysis/fibrinolysis |
Plasmin · tPA/urokinase · PAI-1/2 · α2-AP · α2-macroglobulin · TAFI
|
|
|
|
cell/phys (coag, heme, ), csfs
|
|
drug (B1/2/3+5+6), btst, trns
|
|
|
|
|
Endopeptidases: serine proteases/serine endopeptidases (EC 3.4.21) |
|
| Digestive enzymes |
Enteropeptidase · Trypsin · Chymotrypsin · Elastase (Neutrophil, Pancreatic) |
|
| Coagulation |
factors: Thrombin · Factor VIIa · Factor IXa · Factor Xa · Factor XIa · Factor XIIa · Kallikrein ( PSA, KLK1, KLK2, KLK3, KLK4, KLK5, KLK6, KLK7, KLK8, KLK9, KLK10, KLK11, KLK12, KLK13, KLK14, KLK15)
fibrinolysis: Plasmin · Plasminogen activator (Tissue plasminogen activator · Urinary plasminogen activator)
|
|
| Complement system |
Factor B · Factor D · Factor I · MASP (MASP1, MASP2) · C3-convertase
|
|
| Other immune system |
Chymase · Granzyme · Tryptase · Proteinase 3/Myeloblastin
|
|
| Venombin |
Ancrod · Batroxobin
|
|
| Other |
Acrosin · Prolyl endopeptidase · Pronase · Proprotein convertases (1, 2) · Reelin · Subtilisin/Furin · Streptokinase · S1P · Cathepsin (A, G)
|
|
| enzm: 1.1/////////11///, //////7/, 2.7.10, , 3.1/2//4//6/, , 3.4.21/22/23/, 4.1/////, ////, // |
|
|
Antigens: Autoantigens |
|
| Dehydrogenase |
Branched-chain alpha-keto acid dehydrogenase complex · Oxoglutarate dehydrogenase · Pyruvate dehydrogenase
|
|
| Transglutaminase |
Epidermal transglutaminase · Tissue transglutaminase
|
|
| Nucleoporins |
NUP35 · NUP37 · NUP43 · NUP50 · NUP54 · NUP62 · NUP85 · NUP88 · NUP93 · NUP98 · NUP107 · NUP133 · NUP153 · NUP155 · NUP160 · NUP188 · NUP210 · NUP205 · NUP214
|
|
| Other |
Acetylcholine receptor · Actin · Apolipoprotein H · Cardiolipin · Centromere · Filaggrin(Citrullinate) · Gangliosides · Sp100 nuclear antigen · Thrombin · Topoisomerase |
|
|
|
cell/phys/auag/auab/comp,imrc,tcrp
|
|
|
|
|
|
|
Antihemorrhagics (B02) |
|
Hemostatics
(coagulation) |
|
Systemic
|
|
|
Phytomenadione (K1) · Menadione (K3)
|
|
|
|
intrinsic: IX/Nonacog alfa · VIII
extrinsic: VII/Eptacog alfa
common: X · II/Thrombin · I/Fibrinogen
|
|
|
Other systemic
|
|
|
|
|
Local
|
Absorbable gelatin sponge · Oxidized cellulose · Tetragalacturonic acid hydroxymethylester · Thrombin · Collagen · Calcium alginate · Epinephrine/Adrenalone |
|
|
| Antifibrinolytics |
amino acids (Aminocaproic acid, Tranexamic acid, Aminomethylbenzoic acid)
serpins (Aprotinin, Alfa1 antitrypsin, C1-inhibitor, Camostat)
|
|
|
|
cell/phys (coag, heme, ), csfs
|
|
drug (B1/2/3+5+6), btst, trns
|
|
|
|