Porphyrin
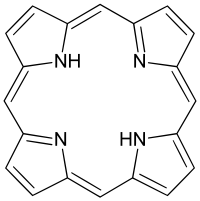

Porphyrins are a group of organic compounds of which many occur in nature. One of the best known is hemoglobin, the pigment in red blood cells. They are heterocyclic macrocycles characterised by the presence of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH-). Porphyrins are aromatic. That is, they obey Hückel's rule for aromaticity, possessing 4n+2 = π electrons (n=4 for the shortest cyclic path) that are delocalized over the macrocycle. The macrocycles, therefore, are highly-conjugated systems. Consequently, thay have very intense absorption in the visible region and therefore are deeply colored; the name porphyrin comes from a Greek word for purple. The macrocycle has 26 pi electrons in total. The parent porphyrin is porphine, and substituted porphines are called porphyrins.
Contents |
Porphyrins are the conjugate acids of ligands that bind metals to form complexes. The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown:
- H2porphyrin + [MLn]2+ → M(porphyrinate)Ln-4 + 4 L + 2 H+
A porphyrin without metal in its cavity is a free base. Some iron-containing porphyrins are called hemes. Heme-containing proteins, or hemoproteins, are found extensively in nature. Hemoglobin and myoglobin are two O2-binding proteins that contain iron porphyrins.
Related to porphyrins are several other heterocycles, including corrins, chlorins, bacteriochlorophylls, and corphins. Chlorins (2,3-dihydroporphyrin) are more reduced, contain more hydrogen than porphyrins, and feature a pyrroline subunit. This structure occurs in chlorophyll. Replacement of two of the four pyrrolic subunits with pyrrolinic subunits results in either a bacteriochlorin (as found in some photosynthetic bacteria) or an isobacteriochlorin, depending on the relative positions of the reduced rings. Some porphyrin derivatives follow Hückel's rule, but most do not.
Synthesis
Biosynthesis
The "committed step" for porphyrin biosynthesis is the formation of D-aminolevulinic acid (dALA) by the reaction of the amino acid glycine and succinyl-CoA, from the citric acid cycle. Two molecules of dALA combine to give porphobilinogen (PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX is combined with iron to form heme. Bile pigments are the breakdown products of heme.
The following scheme summarizes the biosynthesis of porphyrins, with references by EC number and the OMIM database. The porphyria associated with the deficiency of each enzyme is also shown:
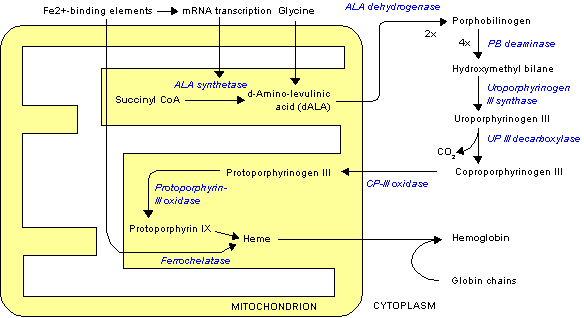
| Enzyme | substrate | Product | Chromosome | EC | OMIM | porphyria |
| ALA synthase | Glycine, succinyl CoA | D-Aminolevulinic acid | 3p21.1 | 2.3.1.37 | 125290 | none |
| ALA dehydratase | D-Aminolevulinic acid | Porphobilinogen | 9q34 | 4.2.1.24 | 125270 | ALA-Dehydratase deficiency |
| PBG deaminase | Porphobilinogen | Hydroxymethyl bilane | 11q23.3 | 2.5.1.61 | 176000 | acute intermittent porphyria |
| Uroporphyrinogen III synthase | Hydroxymethyl bilane | Uroporphyrinogen III | 10q25.2-q26.3 | 4.2.1.75 | 606938 | congenital erythropoietic porphyria |
| Uroporphyrinogen III decarboxylase | Uroporphyrinogen III | Coproporphyrinogen III | 1p34 | 4.1.1.37 | 176100 | porphyria cutanea tarda |
| Coproporphyrinogen III oxidase | Coproporphyrinogen III | Protoporphyrinogen IX | 3q12 | 1.3.3.3 | 121300 | coproporphyria |
| Protoporphyrinogen oxidase | Protoporphyrinogen IX | Protoporphyrin IX | 1q22 | 1.3.3.4 | 600923 | variegate porphyria |
| Ferrochelatase | Protoporphyrin IX | Heme | 18q21.3 | 4.99.1.1 | 177000 | erythropoietic protoporphyria |
Laboratory synthesis

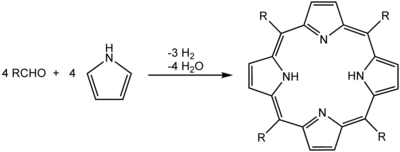
One of the more common syntheses for porphyrins is based on work by Paul Rothemund.[1][2] His techniques underpin more modern syntheses such as those described by Adler and Longo.[3] The synthesis of simple porphyrins such as meso-tetraphenylporphyrin (H2TPP) is also commonly done in university teaching labs.[4] It can be done by heating an equimolar mixture of pyrrole and benzaldehyde in refluxing propionic acid for about an hour.
In this method, porphyrins are assembled from pyrrole and substituted aldehydes. Acidic conditions are essential; formic acid, acetic acid, and propionic acid are typical reaction solvents, or p-toluenesulfonic acid can be used with a non-acidic solvent. Lewis acids such as boron trifluoride etherate and ytterbium triflate have also been known to catalyse porphyrin formation. A large amount of side-product is formed and is removed, usually by recrystallization or chromatography.
Applications
Although natural porphyrin complexes are essential for life, synthetic porphyrins and their complexes have limited utility. Complexes of meso-tetraphenylporphyrin, e.g., the iron(III) chloride complex (TPPFeCl), catalyze a variety of reactions in organic synthesis, but none of them are of practical value. Porphyrin-based compounds are of interest in molecular electronics and supramolecular building blocks. Phthalocyanines, which are structurally related to porphyrins, are used in commerce as dyes and catalysts. Synthetic porphyrin dyes that are incorporated in the design of solar cells are the subject of ongoing research. See Dye-sensitized solar cells.
In 2008 the corporation Destiny Pharma reported successful clinical trials of an intra-nasally applied porphyrin XF-73 against methicillin-resistant Staphylococcus aureus.[5]
Supramolecular chemistry

Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host-guest complex that was constructed from a macrocycle composed of four porphyrins.[6] A guest-free base porphyrin is bound to the center by coordination with its four pyridine substituents.
Organic geochemistry
The field of organic geochemistry, the study of the impacts and processes that organisms have had on the Earth, had its origins in the isolation of porphyrins from petroleum. This finding helped establish the biological origins of petroleum. Petroleum is sometimes "fingerprinted" by analysis of trace amounts of nickel and vanadyl porphyrins.
Chlorophyl is a magnesium porphyrin, heme is a iron porphyrin but they are not present in petroleum. On the other hand, nickel and vanadyl porphyrins could be related to catalytic molecules from bacteria that feed primordial hydrocarbons.
See also
- A porphyrin-related disease: porphyria
- Porphyrin coordinated to iron: heme
- Porphyrin coordinated to magnesium: chlorophyll
- The one-carbon-shorter analogues: corroles, including vitamin B12 which is coordinated to a cobalt
- Corphins, the highly-reduced porphyrin coordinated to nickel that binds the F430 active site in methyl coenzyme M reductase (MCR)
- Nitrogen-substituted porphyrins: phthalocyanine
Gallery
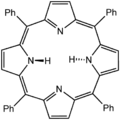 Lewis structure for meso-tetraphenylporphyrin |
UV-vis readout for meso-tetraphenylporphyrin |
References
- ↑ P. Rothemund (1936). "A New Porphyrin Synthesis. The Synthesis of Porphin". J. Am. Chem. Soc. 58 (4): 625–627. doi:10.1021/ja01295a027.
- ↑ P. Rothemund (1935). "Formation of Porphyrins from Pyrrole and Aldehydes". J. Am. Chem. Soc. 57 (10): 2010–2011. doi:10.1021/ja01313a510.
- ↑ A. D. Adler, F. R. Longo, J. D. Finarelli, J. Goldmacher, J. Assour and L. Korsakoff (1967). "A simplified synthesis for meso-tetraphenylporphine". J. Org. Chem. 32 (2): 476–476. doi:10.1021/jo01288a053.
- ↑ Falvo, RaeAnne E.; Mink, Larry M.; Marsh, Diane F.. "Microscale Synthesis and 1H NMR Analysis of Tetraphenylporphyrins". J. Chem. Educ. 1999 (76): 237.
- ↑ "Hope for new way to destroy MRSA". BBC News. 2008-05-23. http://news.bbc.co.uk/2/hi/health/7406832.stm. Retrieved 2010-05-22.
- ↑ 6.0 6.1 Sally Anderson, Harry L. Anderson, Alan Bashall, Mary McPartlin, Jeremy K. M. Sanders (1995). "Assembly and Crystal Structure of a Photoactive Array of Five Porphyrins". Angew. Chem., Int. Ed. Engl. 34: 1096–1099. doi:10.1002/anie.199510961.
External links
- Journal of Porphyrins and Phthalocyanines
- Handbook of Porphyrin Science
- Porphynet - an informative site about porphyrins and related structures
|
|||||||||||||||||||||||||||||||||||