Polyadenylation

Polyadenylation is the addition of a poly(A) tail to an RNA molecule. The poly(A) tail consists of multiple adenosine monophosphates; in other words, it is a stretch of RNA which only has Adenine bases. In eukaryotes, polyadenylation is part of the process that produces mature messenger RNA (mRNA) for translation. It therefore forms part of the larger process of gene expression.
The process of polyadenylation begins as the transcription of a gene finishes. The 3'-most segment of the newly-made RNA is first cleaved off by a set of proteins; these proteins then synthesise the poly(A) tail at the RNA's 3' end. In some genes these proteins may add a poly(A) tail at any one of several possible sites, polyadenylation can therefore produce more than one transcript from a single gene, similar to alternative splicing.[1]
The poly(A) tail is important for the nuclear export, translation and stability of mRNA. The tail is shortened over time and when it is short enough, the mRNA is enzymatically degraded.[2] However, in a few cell types, mRNAs with short poly(A) tails are stored for later activation by re-polyadenylation in the cytosol.[3] In contrast, when polyadenylation occurs in bacteria, it promotes RNA degradation.[4] This is also sometimes the case for eukaryotic non-coding RNAs.[5] The wide distribution of polyadenylation among living organisms indicates that this process evolved early in the history of life on Earth.
Contents |
Primer on RNA
- For further information, see RNA and Messenger RNA
RNAs are a type of large biological molecules, whose individual building blocks are called nucleotides. The name poly(A) tail (for polyadenylic acid tail)[6] reflects the way RNA nucleotides are abbreviated, with a letter for the base the nucleotide contains (A for adenine, C for cytosine, G for guanine and U for uracil). RNAs are produced (transcribed) from a DNA template. By convention, RNA sequences are written in a 5' to 3' direction. The 5' end is the part of the RNA molecule that is transcribed first, and the 3' end is transcribed last. The 3' end is also where the poly(A) tail is found on polyadenylated RNAs.[1][7]
Messenger RNA (mRNA) is RNA that has a coding region that acts as a template for protein synthesis (translation). The rest of the mRNA, the untranslated regions, tune how active the mRNA is.[8] There are also many RNAs that are not translated, called non-coding RNAs. Like the untranslated regions, many of these non-coding RNAs have regulatory roles.[9]
Nuclear polyadenylation
Function
In nuclear polyadenylation, a poly(A) tail is added to an RNA at the end of transcription. On mRNAs, the poly(A) tail protects the mRNA molecule from enzymatic degradation in the cytoplasm and aids in transcription termination, export of the mRNA from the nucleus, and translation.[2] Almost all eukaryotic mRNAs are polyadenylated,[10] with the exception of animal replication-dependent histone mRNAs.[11] These are the only mRNAs in eukaryotes that lack a poly(A) tail, ending instead in a stem-loop structure followed by a purine-rich sequence, termed histone downstream element, that directs where the RNA is cut so that the 3' end of the histone mRNA is formed.[12]
Many eukaryotic non-coding RNAs are always polyadenylated at the end of transcription. There are small RNAs where the poly(A) tail is only seen in intermediary forms and not in the mature RNA as the ends are removed during processing, notably microRNAs.[13][14] But for many long noncoding RNAs – a seemingly large group of regulatory RNAs that for example includes the RNA Xist which mediates X chromosome inactivation – a poly(A) tail is part of the mature RNA.[15]
Mechanism
| Proteins involved:[10] CPSF: cleavage/polyadenylation specificity factor |
The polyadenylation machinery in the nucleus of eukaryotes works on products of RNA polymerase II, such as precursor mRNA. Here, a multi-protein complex (see components on the right) cleaves the 3'-most part of a newly produced RNA and polyadenylates the end produced by this cleavage. The cleavage is catalysed by the enzyme CPSF[11] and occurs 10–30 nucleotides downstream of its binding site.[16] This site is often the sequence AAUAAA on the RNA, but variants of it exist that bind more weakly to CPSF.[17] Two other proteins add specificity to the binding to an RNA: CstF and CFI. CstF binds to a GU-rich region further downstream of CPSF's site.[18] CFI recognises a third site on the RNA (a set of UGUAA sequences in mammals) and can recruit CPSF even if the AAUAAA sequence is missing.[19][20] The polyadenylation signal – the sequence motif recognised by the RNA cleavage complex – varies between groups of eukaryotes. Most human polyadenylation sites contain the AAUAAA sequence,[18] but this sequence is less common in plants and fungi.[21]
The RNA is cleaved right after transcription, as CstF also binds to RNA polymerase II.[22] Cleavage also involves the protein CFII, unknown how.[23] The cleavage site associated with a polyadenylation signal can vary up to some 50 nucleotides.[24] When the RNA is cleaved, polyadenylation starts, catalysed by polyadenylate polymerase. Polyadenylate polymerase builds the poly(A) tail by adding adenosine monophosphate units from adenosine triphosphate to the RNA, cleaving off pyrophosphate.[25] Another protein, PAB2, binds to the new, short poly(A) tail and increases the affinity of polyadenylate polymerase for the RNA. When the poly(A) tail is approximately 250 nucleotides long the enzyme can no longer bind to CPSF and polyadenylation stops, thus determing the length of the poly(A) tail.[26][27] CPSF is in contact with RNA polymerase II, allowing it to tell the polymerase to terminate transcription.[28][29] The polyadenylation machinery is also physically linked to the spliceosome, a complex that removes introns from RNAs.[20]
-binding_Protein_.jpg)
Downstream effects
The poly(A) tail acts as the binding site for poly(A)-binding protein. Poly(A)-binding protein promotes export from the nucleus and translation, and inhibits degradation.[30] This protein binds to the poly(A) tail prior to mRNA export from the nucleus and in yeast also recruits poly(A) nuclease, an enzyme that shortens the poly(A) tail and allows the export of the mRNA. Poly(A)-binding protein is exported to the cytoplasm with the RNA. mRNAs that are not exported are degraded by the exosome.[31][32] Poly(A)-binding protein also can bind to, and thus recruit, several proteins that affect translation,[31] one of these is initiation factor-4G which in turn recruits the 40S ribosomal subunit.[33] However, a poly(A) tail is not required for the translation of all mRNAs.[34]
Deadenylation
In eukaryotic somatic cells, the poly(A) tail of most mRNAs in the cytoplasm gradually get shorter, and mRNAs with shorter poly(A) tail are translated less and degraded sooner.[35] However, it can take many hours before an mRNA is degraded.[36] This deadenylation and degradation process can be accelerated by microRNAs complementary to the 3' untranslated region of an mRNA.[37] In immature egg cells, mRNAs with shortened poly(A) tails are not degraded, but are instead stored without being translated. They are then activated by cytoplasmic polyadenylation after fertilisation, during egg activation.[38]
In animals, poly(A) ribonuclease (PARN) can bind to the 5' cap and remove nucleotides from the poly(A) tail. The level of access to the 5' cap and poly(A) tail is important in controlling how soon the mRNA is degraded. PARN deadenylates less if the RNA is bound by the initiation factors 4E (at the 5' cap) and 4G (at the poly(A) tail), so this is why translation reduces deadenylation. The rate of deadenylation may also be regulated by RNA-binding proteins. Once the poly(A) tail is removed, the decapping complex removes the 5' cap, leading to a degradation of the RNA. Several other enzymes that seem to be involved in deadenylation have been identified in yeast.[39]
Alternative polyadenylation
Many protein-coding genes have more than one polyadenylation site, so a gene can code for several mRNAs that differ in their 3' end.[21][40][41] Since alternative polyadenylation changes the length of the 3' untranslated region, it can change which binding sites for microRNAs the 3' untranslated region contains.[16][42] MicroRNAs tend to repress translation and promote degradation of the mRNAs they bind to, although there are examples of microRNAs that stabilise transcripts.[43][44] Alternative polyadenylation can also shorten the coding region, thus making the mRNA code for a different protein,[45][46] but this is much less common than just shortening the 3' untranslated region.[21]
The choice of poly(A) site depends on the expression of the proteins that take part in polyadenylation. For example, the expression of CstF-64, a subunit of cleavage stimulatory factor (CstF), increases in macrophages in response to lipopolysaccharides (a group of bacterial compounds that trigger an immune response). This results in the selection of weak poly(A) sites and thus shorter transcripts. This removes regulatory elements in the 3' untranslated regions of mRNAs for defense-related products like lysozyme and TNF-α. These mRNAs then have longer half-lives and produce more of these proteins.[47] RNA-binding proteins other than those in the polyadenylation machinery can also affect whether a polyadenyation site is used,[48][49][50] as can DNA methylation near the polyadenylation signal.[51]
Cytoplasmic polyadenylation
There is polyadenylation in the cytosol of some animal cell types, namely in the germ line, during early embryogenesis and in post-synaptic sites of nerve cells. This lengthens the poly(A) tail of an mRNA with a shortened poly(A) tail, so that the mRNA will be translated.[52][53] These shortened poly(A) tails are often less than 20 nucleotides, and are lengthened to around 80–150 nucleotides.[3]
In the early mouse embryo, cytoplasmic polyadenylation of maternal RNAs from the egg cell allows the cell to survive and grow even though transcription does not start until the middle of the 2-cell stage (4-cell stage in human).[54][55] In the brain, cytoplasmic polyadenylation is active during learning and could to play a role in long-term potentiation, which is the strengthening of the signal transmission from a nerve cell to another in response to nerve impulses and is important for learning and memory formation.[3][56]
Cytoplasmic polyadenylation requires the RNA-binding proteins CPSF and CPEB, and can involve other RNA-binding proteins like Pumilio.[57] Depending on the cell type, the polymerase can be the same type of polyadenylate polymerase (PAP) that is used in the nuclear process, or the cytoplasmic polymerase GLD-2.[58]
Tagging for degradation in eukaryotes
For many non-coding RNAs, including tRNA, rRNA, snRNA and snoRNA, polyadenylation is a way of marking the RNA for degradation, in at least yeast.[59] This polyadenylation is done in the nucleus by the TRAMP complex, which adds a tail that is around 40 nucleotides long to the 3' end.[60] The RNA is then degraded by the exosome.[61] Poly(A) tails have also been found on human rRNA fragments, both the form of homopolymeric (A only) and heterpolymeric (mostly A) tails.[62]
In prokaryotes and organelles
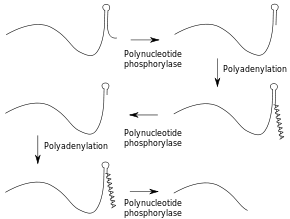
In many bacteria, both mRNAs and non-coding RNAs can be polyadenylated. This poly(A) tail promotes degradation by the degradosome, which contains two RNA-degrading enzymes: polynucleotide phosphorylase and RNase E. Polynucleotide phosphorylase binds to the 3' end of RNAs and the 3' extension provided by the poly(A) tail allows it to bind to the RNAs whose secondary structure would otherwise block the 3' end. Successive rounds of polyadenylation and degradation of the 3' end by polynucleotide phosphorylase allows the degradosome to overcome these secondary structures. The poly(A) tail can also recruit RNases that cut the RNA in two.[63] These bacterial poly(A) tails are about 30 nucleotides long.[64]
In as different groups as animals and trypanosomes, the mitochondria contain both stabilising and destabilising poly(A) tails. Destabilising polyadenylation targets both mRNA and noncoding RNAs. The poly(A) tails are 43 nucleotides long on average. The stabilising ones start at the stop codon, and without them the stop codon (UAA) is not complete as the genome only encodes the U or UA part. Plant mitochondria only have destabilising polyadenylation, and yeast mitochondria have no polyadenylation at all.[65]
While many bacteria and mitochondria have polyadenylate polymerases, they also have another type of polyadenylation, performed by polynucleotide phosphorylase itself. This enzyme is found in bacteria,[66] mitochondria,[67] plastids[68] and as a constituent of the archeal exosome (in those archaea that have an exosome).[69] It can synthesise a 3' extension where the vast majority of the bases are adenines. Like in bacteria, polyadenylation by polynucleotide phosphorylase promotes degradation of the RNA in plastids[70] and likely also archaea.[65]
Evolution
Although polyadenylation is seen in almost all organisms, it is not universal.[71][72] However, the wide distribution of this modification and the fact that it is present in organisms from all three domains of life implies that the last universal common ancestor of all living organisms probably had some form of polyadenylation system.[64] A few organisms do not polyadenylate mRNA, which implies that they have lost their polyadenylation machineries during evolution. Although no examples of eukaryotes that lack polyadenylation are known, mRNAs from the bacterium Mycoplasma gallisepticum and the salt-tolerant archaean Haloferax volcanii lack this modification.[73][74]
The most ancient polyadenylating enzyme is polynucleotide phosphorylase. This enzyme is part of both the bacterial degradosome and archaeal exosome,[75] two closely related complexes that recycle RNA into nucleotides. This enzyme degrades RNA by attacking the bond between the 3'-most nucleotides with a phosphate, breaking off a diphosphate nucleotide. This reaction is reversible, and so the enzyme can also extend RNA with more nucleotides. The heteropolymeric tail added by polynucleotide phosphorylase is very rich in adenine. The choice of adenine is most likely the result of higher ADP concentrations than other nucleotides as a result of using ATP as an energy currency, making it more likely to be incorporated in this tail in early lifeforms. It has been suggested that the involvement of adenine-rich tails in RNA degradation prompted the later evolution of polyadenylate polymerases (the enzymes that produce poly(A) tails with no other nucleotides in them).[76]
Polyadenylate polymerases are not as ancient. They have separately evolved in both bacteria and eukaryotes from CCA-adding enzyme, which is the enzyme that completes the 3' ends of tRNAs. Its catalytic domain is homologous to that of other polymerases.[61] Likely, the horizontal transfer of bacterial CCA-adding enzyme to eukaryotes allowed the archaeal-like CCA-adding enzyme to switch function to a poly(A) polymerase.[64] Some lineages, like archaea and cyanobacteria, never evolved a polyadenylate polymerase.[76]
History
Polyadenylation was first identified in 1960 as an enzymatic activity in extracts made from cell nuclei that could polymerise ATP, but not ADP, into polyadenine.[77][78] Although identified in many types of cells, this activity had no known function until 1971, when poly(A) sequences were found in mRNAs.[79][80] Initially, the only function of these sequences was thought to be protection of the 3' end of the RNA from nucleases, but later the specific roles of polyadenylation in nuclear export and translation were identified. The polymerases responsible for polyadenylation were first purified and characterized in the 1960s and 1970s, but the large number of accessory proteins that control this process were only discovered in the early 1990s.[79]
See also
- Simian virus 40 late polyadenylation signal (SVLPA)
References
- ↑ 1.0 1.1 Proudfoot NJ, Furger A, Dye MJ (2002). "Integrating mRNA processing with transcription". Cell 108 (4): 501–12. doi:10.1016/S0092-8674(02)00617-7. PMID 11909521. http://linkinghub.elsevier.com/retrieve/pii/S0092867402006177.
- ↑ 2.0 2.1 Guhaniyogi J, Brewer G (2001). "Regulation of mRNA stability in mammalian cells". Gene 265 (1-2): 11–23. doi:10.1016/S0378-1119(01)00350-X. PMID 11255003. http://linkinghub.elsevier.com/retrieve/pii/S037811190100350X.
- ↑ 3.0 3.1 3.2 Richter JD (1 June 1999). "Cytoplasmic polyadenylation in development and beyond". Microbiol. Mol. Biol. Rev. 63 (2): 446–56. PMID 10357857. PMC 98972. http://mmbr.asm.org/cgi/pmidlookup?view=long&pmid=10357857.
- ↑ Steege DA (2000). "Emerging features of mRNA decay in bacteria". RNA 6 (8): 1079–90. doi:10.1017/S1355838200001023. PMID 10943888. PMC 1369983. http://www.rnajournal.org/cgi/pmidlookup?view=long&pmid=10943888.
- ↑ Anderson JT (2005). "RNA turnover: unexpected consequences of being tailed". Curr. Biol. 15 (16): R635–8. doi:10.1016/j.cub.2005.08.002. PMID 16111937.
- ↑ Stevens A (1963). "Ribonucleic acids-biosynthesis and degradation". Annu. Rev. Biochem. 32: 15–42. doi:10.1146/annurev.bi.32.070163.000311. PMID 14140701.
- ↑ Nelson DL, Cox MM. Lehninger Principles of biochemistry, 2nd ed., chapter 8. W.H. Freeman.
- ↑ Abaza I, Gebauer F (2008). "Trading translation with RNA-binding proteins". RNA 14 (3): 404–9. doi:10.1261/rna.848208. PMID 18212021.
- ↑ Mattick JS, Makunin IV (2006). "Non-coding RNA". Hum. Mol. Genet. 15 Spec No 1: R17–29. doi:10.1093/hmg/ddl046. PMID 16651366. http://hmg.oxfordjournals.org/cgi/content/full/15/suppl_1/R17.
- ↑ 10.0 10.1 Hunt AG, Xu R, Addepalli B, et al. (2008). "Arabidopsis mRNA polyadenylation machinery: Comprehensive analysis of protein-protein interactions and gene expression profiling". BMC Genomics 9: 220. doi:10.1186/1471-2164-9-220. PMID 18479511.
- ↑ 11.0 11.1 Dávila López M, Samuelsson T (2008). "Early evolution of histone mRNA 3' end processing". RNA 14 (1): 1–10. doi:10.1261/rna.782308. PMID 17998288.
- ↑ William F Marzluff, Preetam Gongidi, Keith R Woods, Jianping Jin, Lois J. Maltais (2002). "The Human and Mouse Replication-Dependent Histone Genes". Genomics 80 (5): 487–98. doi:10.1006/geno.2002.6850 (inactive 2010-03-18). PMID 12408966.
- ↑ Saini HK, Griffiths-Jones S, Enright AJ (2007). "Genomic analysis of human microRNA transcripts". Proc. Natl. Acad. Sci. U.S.A. 104 (45): 17719–24. doi:10.1073/pnas.0703890104. PMID 17965236.
- ↑ Yoshikawa M, Peragine A, Park MY, Poethig RS (2005). "A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis". Genes Dev. 19 (18): 2164–75. doi:10.1101/gad.1352605. PMID 16131612.
- ↑ Amaral PP, Mattick JS (2008). "Noncoding RNA in development". Mammalian Genome 19 (7-8): 454–92. doi:10.1007/s00335-008-9136-7. PMID 18839252.
- ↑ 16.0 16.1 Liu D, Brockman JM, Dass B, et al. (2007). "Systematic variation in mRNA 3'-processing signals during mouse spermatogenesis". Nucleic Acids Res. 35 (1): 234–46. doi:10.1093/nar/gkl919. PMID 17158511.
- ↑ Lutz CS (2008). "Alternative Polyadenylation: A Twist on mRNA 3' End Formation". ACS chemical biology 3 (10): 609–17. doi:10.1021/cb800138w. PMID 18817380.
- ↑ 18.0 18.1 Beaudoing E, Freier S, Wyatt JR, Claverie JM, Gautheret D (2000). "Patterns of variant polyadenylation signal usage in human genes". Genome Res. 10 (7): 1001–10. doi:10.1101/gr.10.7.1001. PMID 10899149. PMC 310884. http://www.genome.org/cgi/pmidlookup?view=long&pmid=10899149.
- ↑ Venkataraman K, Brown KM, Gilmartin GM (2005). "Analysis of a noncanonical poly(A) site reveals a tripartite mechanism for vertebrate poly(A) site recognition". Genes Dev. 19 (11): 1315–27. doi:10.1101/gad.1298605. PMID 15937220.
- ↑ 20.0 20.1 Millevoi S, Loulergue C, Dettwiler S, et al. (2006). "An interaction between U2AF 65 and CF I(m) links the splicing and 3' end processing machineries". EMBO J. 25 (20): 4854–64. doi:10.1038/sj.emboj.7601331. PMID 17024186.
- ↑ 21.0 21.1 21.2 Shen Y, Ji G, Haas BJ, et al. (2008). "Genome level analysis of rice mRNA 3'-end processing signals and alternative polyadenylation". Nucleic Acids Res. 36 (9): 3150–61. doi:10.1093/nar/gkn158. PMID 18411206.
- ↑ Glover-Cutter K, Kim S, Espinosa J, Bentley DL (2008). "RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes". Nat. Struct. Mol. Biol. 15 (1): 71–8. doi:10.1038/nsmb1352. PMID 18157150.
- ↑ Stumpf G, Domdey H (1996). "Dependence of yeast pre-mRNA 3'-end processing on CFT1: A sequence homolog of the mammalian AAUAAA binding factor". Science 274 (5292): 1517–20. doi:10.1126/science.274.5292.1517. PMID 8929410.
- ↑ Iseli C, Stevenson BJ, de Souza SJ, et al. (2002). "Long-range heterogeneity at the 3' ends of human mRNAs". Genome Res. 12 (7): 1068–74. doi:10.1101/gr.62002. PMID 12097343.
- ↑ Balbo PB, Bohm A (2007). "Mechanism of poly(A) polymerase: Structure of the enzyme-MgATP-RNA ternary complex and kinetic analysis". Structure 15 (9): 1117–31. doi:10.1016/j.str.2007.07.010. PMID 17850751.
- ↑ Viphakone N, Voisinet-Hakil F, Minvielle-Sebastia L (2008). "Molecular dissection of mRNA poly(A) tail length control in yeast". Nucleic Acids Res. 36 (7): 2418–33. doi:10.1093/nar/gkn080. PMID 18304944.
- ↑ Wahle E (1995). "Poly(A) tail length control is caused by termination of processive synthesis". J. Biol. Chem. 270 (6): 2800–8. doi:10.1074/jbc.270.6.2800 (inactive 2010-03-18). PMID 7852352. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=7852352.
- ↑ Dichtl B, Blank D, Sadowski M, Hübner W, Weiser S, Keller W (2002). "Yhh1p/Cft1p directly links poly(A) site recognition and RNA polymerase II transcription termination". EMBO J. 21 (15): 4125–35. doi:10.1093/emboj/cdf390. PMID 12145212.
- ↑ Nag A, Narsinh K, Martinson HG (2007). "The poly(A)-dependent transcriptional pause is mediated by CPSF acting on the body of the polymerase". Nat. Struct. Mol. Biol. 14 (7): 662–9. doi:10.1038/nsmb1253. PMID 17572685.
- ↑ Coller JM, Gray NK, Wickens MP (1998). "mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation". Genes & development 12 (20): 3226–35. doi:10.1101/gad.12.20.3226. PMID 9784497. PMC 317214. http://www.genesdev.org/cgi/pmidlookup?view=long&pmid=9784497.
- ↑ 31.0 31.1 Siddiqui N, Mangus DA, Chang TC, Palermino JM, Shyu AB, Gehring K (2007). "Poly(A) nuclease interacts with the C-terminal domain of polyadenylate-binding protein domain from poly(A)-binding protein". J. Biol. Chem. 282 (34): 25067–75. doi:10.1074/jbc.M701256200. PMID 17595167. http://www.jbc.org/cgi/content/full/282/34/25067.
- ↑ Vinciguerra P, Stutz F (2004). "mRNA export: an assembly line from genes to nuclear pores". Curr. Opin. Cell Biol. 16 (3): 285–92. doi:10.1016/j.ceb.2004.03.013. PMID 15145353.
- ↑ Gray NK, Coller JM, Dickson KS, Wickens M (2000). "Multiple portions of poly(A)-binding protein stimulate translation in vivo". EMBO J. 19 (17): 4723–33. doi:10.1093/emboj/19.17.4723. PMID 10970864.
- ↑ Meaux S, Van Hoof A (2006). "Yeast transcripts cleaved by an internal ribozyme provide new insight into the role of the cap and poly(A) tail in translation and mRNA decay". RNA 12 (7): 1323–37. doi:10.1261/rna.46306. PMID 16714281.
- ↑ Meijer HA, Bushell M, Hill K, et al. (2007). "A novel method for poly(A) fractionation reveals a large population of mRNAs with a short poly(A) tail in mammalian cells". Nucleic Acids Res. 35 (19): e132. doi:10.1093/nar/gkm830. PMID 17933768.
- ↑ Lehner B, Sanderson CM (2004). "A protein interaction framework for human mRNA degradation". Genome Res. 14 (7): 1315–23. doi:10.1101/gr.2122004. PMID 15231747.
- ↑ Wu L, Fan J, Belasco JG (2006). "MicroRNAs direct rapid deadenylation of mRNA". Proc. Natl. Acad. Sci. U.S.A. 103 (11): 4034–9. doi:10.1073/pnas.0510928103. PMID 16495412.
- ↑ Cui J, Sackton KL, Horner VL, Kumar KE, Wolfner MF (2008). "Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation". Genetics 178 (4): 2017–29. doi:10.1534/genetics.107.084558. PMID 18430932.
- ↑ Wilusz CJ, Wormington M, Peltz SW (2001). "The cap-to-tail guide to mRNA turnover". Nat. Rev. Mol. Cell Biol. 2 (4): 237–46. doi:10.1038/35067025. PMID 11283721.
- ↑ Tian B, Hu J, Zhang H, Lutz CS (2005). "A large-scale analysis of mRNA polyadenylation of human and mouse genes". Nucleic acids research 33 (1): 201–12. doi:10.1093/nar/gki158. PMID 15647503.
- ↑ Danckwardt S, Hentze MW, Kulozik AE (2008). "3' end mRNA processing: molecular mechanisms and implications for health and disease". EMBO J. 27 (3): 482–98. doi:10.1038/sj.emboj.7601932. PMID 18256699.
- ↑ Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB (2008). "Proliferating cells express mRNAs with shortened 3' untranslated regions and fewer microRNA target sites". Science. 320 (5883): 1643–7. doi:10.1126/science.1155390. PMID 18566288.
- ↑ Tili E, Michaille JJ, Calin GA (2008). "Expression and function of micro-RNAs in immune cells during normal or disease state". Int J Med Sci 5 (2): 73–9. PMID 18392144. PMC 2288788. http://www.medsci.org/v05p0073.htm.
- ↑ Ghosh T, Soni K, Scaria V, Halimani M, Bhattacharjee C, Pillai B (2008). "MicroRNA-mediated up-regulation of an alternatively polyadenylated variant of the mouse cytoplasmic {beta}-actin gene". Nucleic Acids Res. 36 (19): 6318–32. doi:10.1093/nar/gkn624. PMID 18835850. PMC 2577349. http://nar.oxfordjournals.org/cgi/content/full/36/19/6318.
- ↑ Alt FW, Bothwell AL, Knapp M, Siden E, Mather E, Koshland M, Baltimore D (1980). "Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends". Cell 20 (2): 293–301. doi:10.1016/0092-8674(80)90615-7. PMID 6771018.
- ↑ Tian B, Pan Z, Lee JY (2007). "Widespread mRNA polyadenylation events in introns indicate dynamic interplay between polyadenylation and splicing.". Genome Research 17 (2): 156–65. doi:10.1101/gr.5532707. PMID 17210931.
- ↑ Shell SA, Hesse C, Morris SM, Milcarek C (2005). "Elevated levels of the 64-kDa cleavage stimulatory factor (CstF-64) in lipopolysaccharide-stimulated macrophages influence gene expression and induce alternative poly(A) site selection". J. Biol. Chem. 280 (48): 39950–61. doi:10.1074/jbc.M508848200. PMID 16207706. http://www.jbc.org/cgi/content/full/280/48/39950.
- ↑ Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, Darnell JC, Darnell RB (2008). "HITS-CLIP yields genome-wide insights into brain alternative RNA processing". Nature 456 (7221): 464. doi:10.1038/nature07488. PMID 18978773.
- ↑ Hall-Pogar T, Liang S, Hague LK, Lutz CS (2007). "Specific trans-acting proteins interact with auxiliary RNA polyadenylation elements in the COX-2 3'-UTR". RNA 13 (7): 1103–15. doi:10.1261/rna.577707. PMID 17507659.
- ↑ Danckwardt S, Kaufmann I, Gentzel M, Foerstner KU, Gantzert AS, Gehring NH, Neu-Yilik G, Bork P, Keller W, Wilm M, Hentze MW, Kulozik AE (2007). "Splicing factors stimulate polyadenylation via USEs at non-canonical 3' end formation signals". EMBO J. 26 (11): 2658–69. doi:10.1038/sj.emboj.7601699. PMID 17464285. PMC 1888663. http://www.nature.com/emboj/journal/v26/n11/full/7601699a.html.
- ↑ Wood AJ, Schulz R, Woodfine K, et al. (2008). "Regulation of alternative polyadenylation by genomic imprinting". Genes Dev. 22 (9): 1141–6. doi:10.1101/gad.473408. PMID 18451104. PMC 2335310. http://genesdev.cshlp.org/content/22/9/1141.full.
- ↑ Meijer HA, Bushell M, Hill K, et al. (2007). "A novel method for poly(A) fractionation reveals a large population of mRNAs with a short poly(A) tail in mammalian cells". Nucleic acids research 35 (19): e132. doi:10.1093/nar/gkm830. PMID 17933768.
- ↑ Jung MY, Lorenz L, Richter JD (2006). "Translational control by neuroguidin, a eukaryotic initiation factor 4E and CPEB binding protein". Molecular and cellular biology 26 (11): 4277–87. doi:10.1128/MCB.02470-05. PMID 16705177.
- ↑ Sakurai T, Sato M, Kimura M (2005). "Diverse patterns of poly(A) tail elongation and shortening of murine maternal mRNAs from fully grown oocyte to 2-cell embryo stages". Biochem. Biophys. Res. Commun. 336 (4): 1181–9. doi:10.1016/j.bbrc.2005.08.250. PMID 16169522.
- ↑ Taft RA (2008). "Virtues and limitations of the preimplantation mouse embryo as a model system". Theriogenology 69 (1): 10–6. doi:10.1016/j.theriogenology.2007.09.032. PMID 18023855.
- ↑ Richter JD (2007). "CPEB: a life in translation". Trends Biochem. Sci. 32 (6): 279–85. doi:10.1016/j.tibs.2007.04.004. PMID 17481902.
- ↑ Piqué M, López JM, Foissac S, Guigó R, Méndez R (2008). "A combinatorial code for CPE-mediated translational control". Cell 132 (3): 434–48. doi:10.1016/j.cell.2007.12.038. PMID 18267074.
- ↑ Benoit P, Papin C, Kwak JE, Wickens M, Simonelig M (2008). "PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila". Development 135 (11): 1969–79. doi:10.1242/dev.021444. PMID 18434412.
- ↑ Reinisch KM, Wolin SL (2007). "Emerging themes in non-coding RNA quality control". Curr. Opin. Struct. Biol. 17 (2): 209–14. doi:10.1016/j.sbi.2007.03.012. PMID 17395456.
- ↑ LaCava J, Houseley J, Saveanu C, et al. (2005). "RNA degradation by the exosome is promoted by a nuclear polyadenylation complex". Cell 121 (5): 713–24. doi:10.1016/j.cell.2005.04.029. PMID 15935758.
- ↑ 61.0 61.1 Martin G, Keller W (2007). "RNA-specific ribonucleotidyl transferases". RNA 13 (11): 1834–49. doi:10.1261/rna.652807. PMID 17872511.
- ↑ Slomovic S, Laufer D, Geiger D, Schuster G (2006). "Polyadenylation of ribosomal RNA in human cells". Nucleic Acids Res. 34 (10): 2966–75. doi:10.1093/nar/gkl357. PMID 16738135.
- ↑ Régnier P, Arraiano CM (2000). "Degradation of mRNA in bacteria: Emergence of ubiquitous features". Bioessays 22 (3): 235–44. doi:10.1002/(SICI)1521-1878(200003)22:3<235::AID-BIES5>3.0.CO;2-2. PMID 10684583.
- ↑ 64.0 64.1 64.2 Anantharaman V, Koonin EV, Aravind L (2002). "Comparative genomics and evolution of proteins involved in RNA metabolism". Nucleic Acids Res. 30 (7): 1427–64. doi:10.1093/nar/30.7.1427. PMID 11917006. PMC 101826. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11917006.
- ↑ 65.0 65.1 Slomovic S, Portnoy V, Liveanu V, Schuster G (2006). "RNA polyadenylation in prokaryotes and organelles; different tails tell different tales". Critical Reviews in Plant Sciences 25 (1): 65–77. doi:10.1080/07352680500391337.
- ↑ Chang SA, Cozad M, Mackie GA, Jones GH (2008). "Kinetics of polynucleotide phosphorylase: comparison of enzymes from Streptomyces and Escherichia coli and effects of nucleoside diphosphates". J. Bacteriol. 190 (1): 98–106. doi:10.1128/JB.00327-07. PMID 17965156.
- ↑ Nagaike T, Suzuki T, Ueda T (2008). "Polyadenylation in mammalian mitochondria: insights from recent studies". Biochim. Biophys. Acta 1779 (4): 266–9. doi:10.1016/j.bbagrm.2008.02.001. PMID 18312863.
- ↑ Walter M, Kilian J, Kudla J (2002). "PNPase activity determines the efficiency of mRNA 3'-end processing, the degradation of tRNA and the extent of polyadenylation in chloroplasts". EMBO J. 21 (24): 6905–14. doi:10.1093/emboj/cdf686. PMID 12486011.
- ↑ Portnoy V, Schuster G (2006). "RNA polyadenylation and degradation in different Archaea; roles of the exosome and RNase R". Nucleic Acids Res. 34 (20): 5923–31. doi:10.1093/nar/gkl763. PMID 17065466.
- ↑ Yehudai-Resheff S, Portnoy V, Yogev S, Adir N, Schuster G (2003). "Domain analysis of the chloroplast polynucleotide phosphorylase reveals discrete functions in RNA degradation, polyadenylation, and sequence homology with exosome proteins". Plant Cell 15 (9): 2003–19. doi:10.1105/tpc.013326. PMID 12953107. PMC 181327. http://www.plantcell.org/cgi/pmidlookup?view=long&pmid=12953107.
- ↑ Sarkar N (1997). "Polyadenylation of mRNA in prokaryotes". Annu. Rev. Biochem. 66: 173–97. doi:10.1146/annurev.biochem.66.1.173. PMID 9242905.
- ↑ Slomovic S, Portnoy V, Schuster G (2008). "Detection and characterization of polyadenylated RNA in Eukarya, Bacteria, Archaea, and organelles". Meth. Enzymol. 447: 501–20. doi:10.1016/S0076-6879(08)02224-6. PMID 19161858.
- ↑ Portnoy V, Evguenieva-Hackenberg E, Klein F, et al. (2005). "RNA polyadenylation in Archaea: not observed in Haloferax while the exosome polynucleotidylates RNA in Sulfolobus". EMBO Rep. 6 (12): 1188–93. doi:10.1038/sj.embor.7400571. PMID 16282984.
- ↑ Portnoy V, Schuster G (2008). "Mycoplasma gallisepticum as the first analyzed bacterium in which RNA is not polyadenylated". FEMS Microbiol. Lett. 283 (1): 97–103. doi:10.1111/j.1574-6968.2008.01157.x. PMID 18399989.
- ↑ Evguenieva-Hackenberg E, Roppelt V, Finsterseifer P, Klug G (2008). "Rrp4 and Csl4 are needed for efficient degradation but not for polyadenylation of synthetic and natural RNA by the archaeal exosome". Biochemistry 47 (50): 13158–68. doi:10.1021/bi8012214. PMID 19053279.
- ↑ 76.0 76.1 Slomovic S, Portnoy V, Yehudai-Resheff S, Bronshtein E, Schuster G (2008). "Polynucleotide phosphorylase and the archaeal exosome as poly(A)-polymerases". Biochim. Biophys. Acta 1779 (4): 247–55. doi:10.1016/j.bbagrm.2007.12.004. PMID 18177749.
- ↑ Edmonds M, Abrams R (1 April 1960). "Polynucleotide biosynthesis: formation of a sequence of adenylate units from adenosine triphosphate by an enzyme from thymus nuclei". J. Biol. Chem. 235 (4): 1142–9. PMID 13819354. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=13819354.
- ↑ Colgan DF, Manley JL (1997). "Mechanism and regulation of mRNA polyadenylation". Genes Dev. 11 (21): 2755–66. doi:10.1101/gad.11.21.2755. PMID 9353246. http://www.genesdev.org/cgi/pmidlookup?view=long&pmid=9353246.
- ↑ 79.0 79.1 Edmonds M (2002). "A history of poly A sequences: from formation to factors to function". Prog. Nucleic Acid Res. Mol. Biol. 71: 285–389. doi:10.1016/S0079-6603(02)71046-5. PMID 12102557.
- ↑ Edmonds M, Vaughan MH, Nakazato H (1971). "Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship". Proc. Natl. Acad. Sci. U.S.A. 68 (6): 1336–40. doi:10.1073/pnas.68.6.1336. PMID 5288383. PMC 389184. http://www.pnas.org/content/68/6/1336.full.pdf+html.
Further reading
- Danckwardt et al. 2008. 3' end mRNA processing: molecular mechanisms and implications for health and disease. EMBO J 27: 482–98.
|
|||||||||||