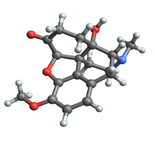
Oxycodone
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| (5R,9R,13S,14S)-4,5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one | |
| Identifiers | |
| CAS number | 76-42-6 |
| ATC code | N02AA05 N02AA55 (in combinations) |
| PubChem | CID 5284603 |
| DrugBank | DB00497 |
| ChemSpider | 4447649 |
| Chemical data | |
| Formula | C18H21NO4 |
| Mol. mass | 315.364 g/mol |
| SMILES | eMolecules & PubChem |
| Synonyms | dihydrohydroxycodeinone, 14-hydroxydihydrocodeinone, 6-deoxy-7,8-dihydro-14-hydroxy-3-O-methyl-6-oxomorphine[1] |
| Pharmacokinetic data | |
| Bioavailability | Up to 87% |
| Protein binding | 45% |
| Metabolism | Hepatic (CYP450: 2D6 substrate) |
| Half-life | 3 - 4.5 hr |
| Excretion | Urine (19% unchanged) |
| Therapeutic considerations | |
| Pregnancy cat. | B/D (prolonged use or in high doses at term) |
| Legal status | Controlled (S8) (AU) Schedule I (CA) ? (UK) Schedule II (US) |
| Dependence liability | Moderate - High |
| Routes | oral, intramuscular, intravenous, intranasal, subcutaneous, transdermal, rectal, epidural[2] |
| |
|
Oxycodone is an opioid analgesic medication synthesized from opium-derived thebaine. It was developed in 1916 in Germany, as one of several new semi-synthetic opioids in an attempt to improve on the existing opiates: morphine, diacetylmorphine (heroin), and codeine.[2]
Oxycodone oral medications are generally prescribed for the relief of moderate to severe pain. Currently it is formulated as single ingredient products or compounded products. Some common examples of compounding are oxycodone with acetaminophen/paracetamol or NSAIDs such as ibuprofen. The formulations are available as generics but are also made under various brand names.
OxyContin is Purdue Pharma's brand for time-release single-ingredient oxycodone oral medication. The manufacturing rights to time-released generic oxycodone are under dispute.
Contents |
Chemistry and nomenclature
Oxycodone's chemical name is derived from codeine. The chemical structures are very similar, differing only in that
- Oxycodone has a hydroxyl group at carbon-14 (codeine has just a hydrogen in its place), hence oxycodone;
- Oxycodone has a 7,8-dihydro feature, whereas codeine has a double bond between those two carbons; and
- Oxycodone has a carbonyl group (as in ketones) in place of the hydroxyl group of codeine, hence the "-one" suffix.
It is also similar to hydrocodone, differing only in that it has a hydroxyl group at carbon-14.[3]
Expanded expression for the compound oxycodone in the academic literature include "dihydrohydroxycodeinone",[1][4][5] "Eucodal",[4][5] "Eukodal",[2][6] "14-hydroxydihydrocodeinone",[1][4] and "Nucodan".[4][5] In a UNESCO convention, the translations of "oxycodone" are oxycodon (Dutch), oxycodone (French), oxicodona (Spanish), والأوآسيكودون (Arabic), 羟考酮 (Chinese), and оксикодон (Russian).[7] The word "oxycodone" should not be confused with "oxandrolone", "oxazepam", "oxybutynin", "oxytocin", or "Roxanol".[8]
History
Freund and Speyer of the University of Frankfurt in Germany first synthesized oxycodone from thebaine in 1916,[9] a few years after the German pharmaceutical company Bayer had stopped the mass production of heroin due to hazardous use, harmful use, and dependence. It was hoped that a thebaine-derived drug would retain the analgesic effects of morphine and heroin with less dependence. To some extent this was achieved, as oxycodone does not have the same immediate effect as heroin or morphine nor does it last as long.
The first clinical use of the drug was documented in 1917.[6] It was first introduced to the US market in May 1939..
The International Narcotics Control Board estimates that 11.5 tons of oxycodone were manufactured worldwide in 1998, which grew to 75.2 tons in 2007.[10] Of all countries, the United States had the highest total consumption of oxycodone in 2007 (82% of the world total of 51.6 tons).[10] In addition, in 2007 the U.S. had the highest per capita consumption of oxycodone, followed by Canada, Denmark, Australia, and Norway.[10]
OxyContin
OxyContin is the brand name of a time-release formula of oxycodone produced by the pharmaceutical company Purdue Pharma.[11] It was approved by the U.S. Food and Drug Administration in 1995 and first introduced to the U.S. market in 1996.[11] By 2001, OxyContin was the best-selling non-generic narcotic pain reliever in the U.S.; 2008 sales in the U.S. totaled $2.5 billion.[12] An analysis of data from the U.S. Drug Enforcement Administration found that retail sales of oxycodone "jumped nearly six-fold between 1997 and 2005."[13] Mundipharma distributes OxyContin in Australia,[14] China,[15] and Europe.[16]
OxyContin is available in 5 mg (blue) tablets in Canada, Australia, France, and the U.K.; 10 mg (white) in Canada, France, the U.S., and the U.K.; 15 mg (grey) in the U.S.; 20 mg (pink) in Canada, France, the U.S., and the U.K.; 30 mg (brown) in the U.S.; 40 mg (yellow) in Canada, France, the U.S., and the U.K.; 60 mg (red) in the U.S.; 80 mg (greenish blue) in Canada, France, the U.S., and the U.K.; and 160 mg (blue) in the U.S., and possibly Canada, and the U.K.[17][18][19] In 2001, Purdue Pharma briefly suspended distribution of 160 mg tablets in the U.S. because of the "possibility of illicit use of tablets of such high strength."[11][20]
Lawsuits concerning generic OxyContin
Purdue has multiple patents for OxyContin, but has been involved in a series of ongoing legal battles on the validity of these patents. On June 7, 2005, the United States Court of Appeals for the Federal Circuit upheld a decision from the previous year that some of Purdue’s patents for OxyContin could not be enforced.[21] This decision allowed and led to the immediate announcement from Endo Pharmaceuticals that they would begin launching a generic version of all four strengths of OxyContin.[22] Purdue, however, had already made negotiations with another pharmaceutical company (IVAX Pharmaceuticals) to distribute their brand OxyContin in a generic form.[22] This contract was severed, and as of October 2005 Watson Pharmaceuticals became the exclusive U.S. distributor of Purdue-manufactured generic versions of OxyContin tablets in 10-, 20-, 40-, and 80-milligram dosages.[23]
On February 1, 2006, the Federal Circuit Court of Appeals issued a decision revising its June 7, 2005, decision.[24] This time the court vacated the lower court's "judgment that the patents-in-suit are unenforceable due to inequitable conduct," and the case was "remanded for further proceedings."[24]
Purdue Pharma has since announced resolution of its infringement suits with Endo,[25] Teva,[26] IMPAX,[27] and Mallinckrodt.[28] Endo and Teva each agreed to cease selling generic forms of OxyContin.[25][26] IMPAX negotiated a temporary, and potentially renewable, license.[27] In 2008, Mallinckrodt Pharmaceuticals reintroduced generic OxyContin in the strengths of 10 mg, 20 mg, 40 mg and 80 mg, which was made possible by a temporary royalties-bearing license with Purdue Pharma that expired in 2009.[28]
Marketing and misbranding
Critics have accused Purdue Pharma of putting profits ahead of public interest by applying "significant political pressure" to attempt to reverse South Carolina's requiring prior approval before a person with Medicaid can receive the drug;[29] for "fail[ing] to adequately warn consumers of the risks" of OxyContin such as dependence;[30] and for promoting the drug "aggressively" and by means such as "promotional beach hats, pedometers and swing-music CDs."[30][31]
In May 2007 Purdue Pharma "agreed to pay $19.5 million" in fines relating to aggressive off-label marketing practices of OxyContin in 26 states and the District of Columbia.[32] In specific, the company encouraged dosing more frequent than the recommended interval of 12 hours, and did not fully disclose the risk of hazardous or harmful use.[32]
Later in May 2007 Purdue Pharma and three of its top executives pleaded guilty in a Virginia federal court to charges that they misbranded OxyContin by representing it to have "less euphoric effect and less abuse potential" than it actually has, and by claiming that people taking the drug at low doses could stop taking it suddenly without symptoms of withdrawal.[33] The FDA had not approved these claims.[34] The company and the executives were to pay $634 million in fines for felony and misdemeanor misbranding.[33]
In October 2007, officials in Kentucky filed a lawsuit against Purdue Pharma for misleading health care providers and consumers "regarding the appropriate uses, risks and safety of OxyContin"; as of mid-2008, however, the case had been "consolidated with other lawsuits into a single multi-litigation suit" in a federal court in New York.[35]
Other preparations
Oxy·IR immediate-release oxycodone tablets from Purdue Pharma in Canada are available in 5, 10, and 20 mg strengths.[18]Effective August 10, 2009, Purdue discontinued manufacture and distribution of OxyIR Capsules. COR is also another generic name for oxycodone. It comes in dosages of 10 mg (COR 106), 20 mg (COR 116) and 30 mg (COR126). OxyNorm is available in 5, 10, and 20 mg capsules, and also as a 5 mg/5 ml liquid in 250 ml bottles in Australia, New Zealand, the Netherlands and the U.K.[36][37][38] In addition, OxyNorm is available in a 10 mg/ml liquid concentrate for oral use in the Netherlands and the U.K., and in a 10 mg/ml solution and 50 mg/ml solution for injection or infusion in New Zealand, the Netherlands and the U.K.[38][39]
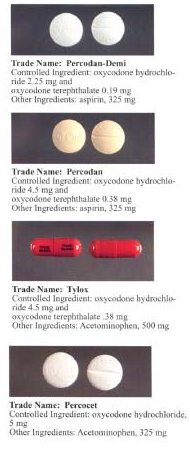
Percocet (oxycodone with paracetamol/acetaminophen) tablets are available in Canada and the U.S. with 2.5, 5, 7.5, and 10 mg of oxycodone and varying amounts of acetaminophen.[40]
Depalgos (oxycodone with paracetamol) tablets are marketed in Italy, with 325 mg Paracetamol and 5, 10, and 20 mg oxycodone. Recent legislation in Italy has made it easier for physicians to prescribe this medication and other opioids to pain patients.[41]
Percodan tablets available in the U.S. contain 4.8355 mg of oxycodone HCl and 325 mg of aspirin.[42]
Proladone suppositories, available in Australia, contain 30 mg of oxycodone pectinate.[43]
Injectable oxycodone hydrochloride or tartrate is available in ampoules and multi-dose vials in many European countries and to a lesser extent various places in the Pacific Rim. For this purpose, the most common trade names are Eukodol and Eucodol. The German-language package insert for an oxycodone injectable indicates that the preferred route of injection is intramuscular.
Roxicodone, a generic oxycodone product designed to have an immediate release effect for rapid pain relief, is available in 5 mg (white), 15 mg (green), and 30 mg (blue) tablets; in a 5 mg per 5 ml oral solution; and in a 20 mg per ml liquid concentrate.[44][45] On March 31, 2009, the U.S. Food and Drug Administration directed Boehringer Ingelheim Roxane and Xanodyne Pharmaceuticals to cease manufacture and distribution of 5 mg Roxicodone tablets in the U.S. because they lacked proper approval.[46]
Targin is a tablet with a prolonged-release oxycodone/naloxone combination.
Clinical use
In a 2008 review written by authors who "are members of advisory boards and speaker panels for Mundipharma," prolonged-release oxycodone (i.e., OxyContin) was found to be superior to placebo in randomized controlled trials concerning diabetic neuropathy, postherpetic neuralgia, osteoarthritis, ambulatory laparoscopic tubal ligation surgery, unilateral total knee arthroplasty, and abdominal/gynaecological surgery.[47]
In 2001, the European Association for Palliative Care recommended that oral hydromorphone or oxycodone, "if available in both normal release and modified release formulations for oral administration," be second-line alternatives to oral morphine for cancer pain.[48] There is no evidence that any opioids are superior to morphine in relieving the pain of cancer, and no controlled trials have shown oxycodone to be superior to morphine.[49] However, switching to an alternative opioid can be useful if adverse effects are troublesome, although the switch can be in either direction, i.e. some patients have fewer adverse effects on switching from morphine to oxycodone and vice versa.
Recreational use
Oxycodone is used very often for recreational use. The drug gives the user a sense of calm and relaxation at certain dosages. It can be injected, smoked, snorted, or taken orally.
Pharmacology
Mechanism of action
A group of Australian researchers has proposed (based on a 1997 study in rats) that oxycodone, unlike morphine (the effect of which is mediated by μ-opioid receptors), acts on κ-opioid receptors.[50] Further research by this group indicates the drug appears to be a κ2b-opioid agonist.[51] However, this has been disputed, primarily on the basis that oxycodone produces effects typical of μ-opioid agonists.[52]
Research by a Japanese group suggests that the effect of oxycodone is mediated by different receptors in different situations. Specifically, in diabetic mice the κ-opioid receptor appears to be involved in the antinociceptive effects of oxycodone,[53] while in non-diabetic mice the μ1-opioid receptor seems to be primarily responsible for these effects.[54]
Absorption
After a dose of conventional oral oxycodone, peak plasma levels of the drug are attained in approximately one hour;[55] in contrast, after a dose of OxyContin (an oral continuous release formulation), peak plasma levels of oxycodone occur in about three hours.[17]
Distribution
Oxycodone in the blood is distributed to skeletal muscle, liver, intestinal tract, lungs, spleen, and brain.[17] Conventional oral preparations of oxycodone start to reduce pain within 10–15 minutes; in contrast, OxyContin starts to reduce pain within 1 hour.[3]
Metabolism
Oxycodone is metabolized to α and β oxycodol; oxymorphone, then α and β oxymorphol and noroxymorphone; and noroxycodone, then α and β noroxycodol and noroxymorphone (N-desmethyloxycodone).[55] These metabolites are true only for humans.[56] As many as six metabolites for oxycodone (14-hydroxydihydromorphinone, 14-hydroxydihydrocodeine, 14-hydroxydihydrocodeinone N-oxide {oxycodone N-oxide}, 14-hydroxydihydroisocodeine, 14-hydroxydihydrocodeine N-oxide, and noroxycodone) have been found in rabbits,[57] several of which are thought to be active metabolites to some extent, although a study using conventional oral oxycodone concluded that oxycodone itself, and not its metabolites, is predominantly responsible for the drug's opioid effects on the brain.[55]
Unlike morphine and hydromorphone, oxycodone is metabolized by the cytochrome P450 enzyme system in the liver, making it vulnerable to drug interactions.[17] Some people are fast metabolizers resulting in reduced analgesic effect but increased adverse effects, while others are slow metabolisers resulting in increased toxicity without improved analgesia.[58][59] The dose of OxyContin must be reduced in patients with reduced hepatic function.[3]
Elimination
Oxycodone and its metabolites are mainly excreted in the urine and sweat; therefore, it accumulates in patients with renal impairment.[3]
Dosage and administration
Oxycodone can be administered orally, intranasally, via intravenous/intramuscular/subcutaneous injection or rectally. The bioavailability of oral administration of OxyContin averages 60–87%, with rectal administration yielding the same results; intranasal varies between individuals with a mean of 46%.[60]
Oxycodone is approximately 1.5–2 times as potent as morphine when administered orally.[61][62] However, 10–15 mg of oxycodone produces an analgesic effect similar to 10 mg of morphine when administered intramuscularly.[63] Therefore, as a parenteral dose, morphine is approximately up to 50% more potent than oxycodone.
There are no comparative trials showing that oxycodone is more effective than any other opioid. In palliative care, morphine remains the gold standard;[49] however, oxycodone can be useful as an alternative opioid if a patient has troublesome adverse effects with morphine.
Side effects
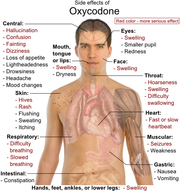
The most commonly reported effects include euphoria, constipation, fatigue, dizziness, nausea, lightheadedness, headache, dry mouth, anxiety, pruritus, and diaphoresis.[65] It has also been claimed to cause dimness in vision due to miosis. Some patients have also experienced loss of appetite, nervousness, abdominal pain, diarrhea, ischuria, dyspnea, and hiccups,[17] although these symptoms appear in less than 5% of patients taking oxycodone. Rarely, the drug can cause impotence, enlarged prostate gland, and decreased testosterone secretion.[66] Compared to morphine, oxycodone causes less respiratory depression, sedation, pruritus, nausea, and euphoria.[67] As a result, it is generally better tolerated than morphine.[68]
In high doses, overdoses, or in patients not tolerant to opiates, oxycodone can cause shallow breathing, bradycardia, cold, clammy skin, apnea, hypotension, miosis (pupil constriction), circulatory collapse, respiratory arrest, and death.[17]
There is a high risk of experiencing severe withdrawal symptoms if a patient discontinues oxycodone abruptly. Therefore therapy should be gradually discontinued rather than abruptly discontinued. People who use oxycodone in a hazardous or harmful fashion are at even higher risk of severe withdrawal symptoms as they tend to use higher than prescribed doses. The symptoms of oxycodone withdrawal are the same as for other opiate based painkillers and may include "anxiety, nausea, insomnia, muscle pain, muscle weakness, fevers, and other flu like symptoms."[69]
Withdrawal symptoms have also been reported in newborns whose mothers had been either injecting or orally taking oxycodone during pregnancy.[70]
Detection in biological fluids
Oxycodone and/or its major metabolites may be quantitated in blood or urine to monitor for abuse, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Many commercial opiate screening tests cross-react appreciably with oxycodone and its metabolites, but chromatographic techniques can easily distinguish oxycodone from the opiates.[71]
Illicit use and diversion
Canada
A study at St. Michael's Hospital and the Institute for Clinical Evaluative Sciences (ICES) in Toronto, found that deaths from opioid pain relievers nearly doubled from 13.7 deaths per million residents in 1991 to 27.2 deaths per million residents in 2004.[72]
United States
Instances of recreational use and diversion of OxyContin have increased in the U.S. beginning in the late 1990s.[73] The slang term hillbilly heroin for OxyContin refers to the occurrence of the "earliest reported cases of Oxycontin abuse" in the U.S. in rural areas such as Appalachia.[74] Diversion of OxyContin in the U.S. may occur through "fraudulent prescriptions, doctor shopping, over-prescribing, and pharmacy theft."[73]
A 2003 study by the Government Accountability Office found four factors that may have contributed to the illicit use and distribution of OxyContin in the U.S.:[11]
- OxyContin contains a large amount of oxycodone compared with other types of oxycodone containing pills.
- OxyContin's warning label said to not crush the controlled-release tablets because of the potential for rapid release of oxycodone, which led to many people crushing the tablets and injecting or snorting the drug.
- By 2001, sales of OxyContin in the U.S. exceeded $1 billion per year.
- People who received prescriptions for OxyContin from across the United States and almost all socioeconomic status have perceived a "profit potential" in selling the pills to drug dealers (e.g., 20 mg of OxyContin could be bought for $2 but sold for $5-20) .
A study published in 2005 examined the prevalence of opiate analgesic use among "recreational drug users and street addicts" as perceived by "key informants" throughout the U.S.; the authors found that non-clinical use of opiates was increasing in general, but that of the drugs studied use of OxyContin "was mentioned most frequently."[75] Purdue Pharma has attempted to reformulate the 10–40 mg strengths of OxyContin to prevent the release of a high percentage of the oxycodone by crushing; however, in 2008 a joint panel convened by the U.S. Food and Drug Administration was "concerned that abusers could find a way to manipulate the new formulation."[76]
One investigation in Boston found that OxyContin was a "gateway" drug for heroin, which addicts turned to as cheaper alternative.[77]
Other countries
The illegal use of OxyContin began in Australia in the early 2000s. By 2007, 51% of a national sample of injection drug users in Australia had reported using oxycodone, and 27% had injected it in the last six months.[78]
Hazardous use, harmful use, and diversion of OxyContin in the U.K. commenced in the early- to mid-2000s.[79] The first known death due to OxyContin overdose in the U.K. occurred in 2002.[80]
Animal studies
Research has shown that the brains of adolescent mice, which were exposed to OxyContin, can sustain lifelong and permanent changes in their reward system.[81][82] It is notable that the vast majority of OxyContin related deaths are attributed to ingesting substantial quantities of oxycodone in combination with another depressant of the central nervous system such as alcohol, barbiturates and related drugs.[83]
Conventions and national laws
Oxycodone is subject to international conventions on narcotic drugs. In addition, oxycodone is subject to national laws that differ by country.
International
The 1931 Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs of the League of Nations included oxycodone.[84] The 1961 Single Convention on Narcotic Drugs of the United Nations, which replaced the 1931 convention, categorized oxycodone in Schedule I.[85] Global restrictions on Schedule I drugs include "limit[ing] exclusively to medical and scientific purposes the production, manufacture, export, import, distribution of, trade in, use and possession of" these drugs; "requir[ing] medical prescriptions for the supply or dispensation of [these] drugs to individuals"; and "prevent[ing] the accumulation" of quantities of these drugs "in excess of those required for the normal conduct of business."[85]
Australia
Oxycodone is in Schedule I (derived from the Single Convention on Narcotic Drugs) of the Commonwealth's Narcotic Drugs Act 1967.[86] In addition, it is in Schedule 8 of the Australian Standard for the Uniform Scheduling of Drugs and Poisons ("Poisons Standard"), meaning that it is a "controlled drug... which should be available for use but require[s] restriction of manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence."[87]
Canada
Oxycodone is a controlled substance under Schedule I of the Controlled Drugs and Substances Act (CDSA).[88] Every person who seeks or obtains from a practitioner either the substance or an authorization to obtain the substance must disclose to that practitioner information on all controlled substances and authorizations for controlled substances obtained from any other practitioner within the preceding 30 days; otherwise, the person may be found "guilty of an indictable offence and liable to imprisonment for a term not exceeding seven years".[88] Anyone possessing the substance for the purpose of trafficking "is guilty of an indictable offence and liable to imprisonment for life".[88]
Germany
The drug is in Appendix III of the Narcotics Act ("Betäubungsmittelgesetz" or BtMG).[89] The law states that only physicians, dentists and veterinarians ("Ärzte, Zahnärzte und Tierärzte") can prescribe oxycodone, and that the federal government can regulate the prescriptions (e.g., by requiring reporting).[89]
Hong Kong
Oxycodone is regulated under Part I of Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance.[90] The penalty for trafficking (Section 4) or manufacturing (Section 6) the substance is a $5,000,000 HKD fine and/or life imprisonment.[90] In Section 8 of the Ordinance, possession of the substance for consumption without licence from the Department of Health is illegal and subject to a $1,000,000 HKD fine and/or 7 years of imprisonment.[90] Per Sections 22-23, only specific health professionals and others (e.g., "a person in charge of a laboratory used for the purposes of research") may possess and supply the substance.[90] Anyone who supplies the substance without a valid prescription can be fined $10,000 HKD according to Section 31.[90]
Singapore
Oxycodone is listed as a Class A drug in the Misuse of Drugs Act of Singapore, which means that offences in relation to the drug attract the most severe level of punishment. A conviction for unauthorized manufacture of the drug attracts a minimum sentence of ten years' imprisonment and corporal punishment of five strokes of the cane, and a maximum sentence of life imprisonment or 30 years' imprisonment and 15 strokes of the cane.[91] The minimum and maximum penalties for unauthorized trafficking in the drug are respectively five years' imprisonment and five strokes of the cane, and 20 years' imprisonment and 15 strokes of the cane.[92]
United Kingdom
Oxycodone is a Class A drug under the Misuse of Drugs Act.[93] For Class A drugs, which are "considered to be the most likely to cause harm," possession without a prescription is punishable by up to seven years in prison, an unlimited fine, or both.[94] Dealing of the drug illegally is punishable by up to life imprisonment, an unlimited fine, or both.[94] In addition, oxycodone is a Schedule 2 drug per the Misuse of Drugs Regulations 2001 which "provide certain exemptions from the provisions of the Misuse of Drugs Act 1971."[95]
United States
Under the Controlled Substances Act, oxycodone is a Schedule II drug because it "has a high potential for abuse," because it "has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions," and because use of the drug "may lead to severe psychological or physical dependence."[96] According to Section 829 of the Act, Schedule II drugs must be dispensed only with the written prescription of a practitioner except in certain situations (e.g., "dispensed directly by a practitioner, other than a pharmacist," or "dispensed upon oral prescription (i.e. telephone)" in "emergency situations").[96] Furthermore, Section 829 specifies that prescriptions for Schedule II drugs cannot be refilled.[96]
As of April 2010 an anti-abuse, controlled release formulation of Oxycontin was approved for sale in the United States. The reformulated OxyContin is intended to prevent tampering with the opioid medication — from being cut, broken, chewed, crushed or dissolved.[97]
See also
- Illegal drug trade
- List of drug-related deaths
- Psychoactive drug
- Recreational drug use
Notes
References
- ↑ 1.0 1.1 1.2 Maryadele J. O'Neil, editor ... (2006). The Merck index (14 ed.). Whitehouse Station, NJ: Merck & Co.. ISBN 978-0-911910-00-1.
- ↑ 2.0 2.1 2.2 Kalso, E (May 2005). "Oxycodone". Journal of Pain and Symptom Management 29 (5S): S47–S56. doi:10.1016/j.jpainsymman.2005.01.010. ISSN 0885-3924. PMID 15907646. http://www.jpsmjournal.com/article/PIIS0885392405000369/fulltext.
- ↑ 3.0 3.1 3.2 3.3 "AHFS Drug Information. Oxycodone (28:08.08) - 382132". American Society of Health-System Pharmacists. March 2008. http://www.ashp.org/mngrphs/ahfs/a382132.htm. Retrieved 2009-03-27.
- ↑ 4.0 4.1 4.2 4.3 Eddy NB (1973). The National Research Council involvement in the opiate problem, 1928–1971. Washington: National Academy of Sciences.
- ↑ 5.0 5.1 5.2 May EL, Jacobson AE (1989). "The Committee on Problems of Drug Dependence: a legacy of the National Academy of Sciences. A historical account". Drug Alcohol Depend 23 (3): 183–218. doi:10.1016/0376-8716(89)90083-5. PMID 2666074.
- ↑ 6.0 6.1 Sunshine A, Olson NZ, Colon A, Rivera J, Kaiko RF, Fitzmartin RD, Reder RF, Goldenheim PD, A; Olson, NZ; Colon, A; Rivera, J; Kaiko, RF; Fitzmartin, RD; Reder, RF; Goldenheim, PD (1 July 1996). "Analgesic efficacy of controlled-release oxycodone in postoperative pain". J Clin Pharmacol 36 (7): 595–603. PMID 8844441. http://jcp.sagepub.com/cgi/reprint/36/7/595. Retrieved 2009-03-25.
- ↑ United Nations Educational, Scientific and Cultural Organization (2005). "International convention against doping in sport" (PDF). http://unesdoc.unesco.org/images/0014/001425/142594m.pdf. Retrieved 2009-04-04.
- ↑ Hicks RW, Becker SC, Cousins DD, eds. (2008) (PDF). MEDMARX data report. A report on the relationship of drug names and medication errors in response to the Institute of Medicine’s call for action. Rockville, MD: Center for the Advancement of Patient Safety, US Pharmacopeia. http://www.usp.org/pdf/EN/medmarx/2008MEDMARXReport.pdf. Retrieved 2009-04-04.
- ↑ Sneader W (2005). Drug discovery: a history. Hoboken, NJ: Wiley. p. 119. ISBN 0471899801.
- ↑ 10.0 10.1 10.2 International Narcotics Control Board (2009) (PDF). Narcotic drugs: estimated world requirements for 2009; statistics for 2007. Report E/INCB/2008/2. New York: United Nations. ISBN 978-92-1-048124-3. http://www.incb.org/pdf/technical-reports/narcotic-drugs/2008/narcotics_drugs_2008.pdf.
- ↑ 11.0 11.1 11.2 11.3 (PDF) Prescription drugs. OxyContin abuse and diversion and efforts to address the problem. Report GAO-04-0110. Washington, DC: U.S. Government Accounting Office. December 2003. http://www.gao.gov/new.items/d04110.pdf. Retrieved 2008-03-28.
- ↑ "Details for Oxycontin". Drugpatentwatch.com. http://drugpatentwatch.com/ultimate/preview/tradename/index.php?query=OXYCONTIN. Retrieved 2010-08-01.
- ↑ Bass F, Associated Press (2007-08-20). "AP: pain medicine use has nearly doubled". Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2007/08/20/AR2007082000147.html. Retrieved 2009-04-16.
- ↑ Nader, Carol (2007-03-02). "Drug users target powerful painkiller". The Age (Melbourne, Australia). http://www.theage.com.au/news/national/drug-users-target-powerful-painkiller/2007/03/01/1172338796110.html. Retrieved 2009-04-09.
- ↑ Yu SY, OxyContin Tablets Postmarketing Surveillance Study Group China (2008). "Postmarketing surveillance study of OxyContin tablets for relieving moderate to severe cancer pain". Oncology 74 (Suppl 1): 46–51. doi:10.1159/000143218. ISSN 0030-2414. PMID 18758197. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=000143218. Retrieved 2009-04-09.
- ↑ Mundipharma International Ltd (2006-11-03). "Mundipharma receives boost to European OxyContin(R) patent position: Teva withdraws challenge from European patent office". PR Newswire. http://www.prnewswire.co.uk/cgi/news/release?id=183302. Retrieved 2009-04-09.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 (PDF) 1. Package insert Oxycontin. Stamford, CT: Purdue Pharma L.P. 2007-11-05. http://www.purduepharma.com/PI/Prescription/Oxycontin.pdf. Retrieved 2009-03-23.
- ↑ 18.0 18.1 (PDF) Product monograph [OxyContin and OxyIR]. Pickering, Ontario: Purdue Pharma. 2008-08-20. http://www.purdue.ca/pdf/2008-08-20%20OxyContin%20and%20OxyIR%20PM_FINAL_ENG.pdf. Retrieved 2009-03-23.
- ↑ "PIL (Patient Information Leaflet). OxyContin tablets". Napp Pharmaceuticals Limited. 2004-05-24. http://emc.medicines.org.uk/medicine/3453/XPIL/OxyContin+tablets/. Retrieved 2009-03-31.
- ↑ Reidy, Maurice Timothy (2001-05-11). "Maker acts on controversial painkiller tablets - 160 mg tablets of Oxycontin drew DEA attention". Hartford Courant.
- ↑ Purdue Pharma L.P. v. Endo Pharms. Inc, 410 F.3d 690 (Fed.Cir. 2005-06-07).
- ↑ 22.0 22.1 Purdue Pharma (2005-06-08). "Purdue comments on Federal Court of Appeal decision on OxyContin patent litigation". Press release. http://www.pharma.com/pressroom/news/20050608.htm. Retrieved 2009-04-11.
- ↑ Purdue Pharma (2005-10-28). "Purdue appoints Watson Pharmaceuticals exclusive distributor of authorized generic versions of OxyContin tablets". Press release. http://www.pharma.com/pressroom/news/20051028.htm. Retrieved 2009-04-11.
- ↑ 24.0 24.1 Purdue Pharma L.P. v. Endo Pharms. Inc., 438 F.3d 1123 (Fed.Cir. 2006-02-01).
- ↑ 25.0 25.1 Purdue Pharma (2006-08-28). "Purdue Pharma L.P. announces resolution of OxyContin patent lawsuit with Endo Pharmaceuticals". Press release. http://www.purduepharma.com/pressroom/news/20060828-1.htm. Retrieved 2009-04-11.
- ↑ 26.0 26.1 Purdue Pharma (2006-10-16). "Purdue Pharma L.P. announces signing of consent judgment ending OxyContin tablets patent lawsuit with Teva Pharmaceuticals". Press release. http://www.purduepharma.com/pressroom/news/20061016.htm. Retrieved 2009-04-11.
- ↑ 27.0 27.1 Purdue Pharma (2007-04-02). "Purdue Pharma L.P. announces agreement to end OxyContin patent lawsuit with IMPAX laboratories". Press release. http://www.purduepharma.com/pressroom/news/20070402.htm. Retrieved 2009-04-11.
- ↑ 28.0 28.1 Purdue Pharma (2009-04-11). "Purdue Pharma L.P. announces resolution of OxyContin patent lawsuit with Mallinckrodt Inc.". Press release. http://www.purduepharma.com/pressroom/news/20080902.htm. Retrieved 2009-04-11.
- ↑ Bauerlein V (2001-09-23). "Popular painkiller mired in controversy" (reprint). The State. http://www.mapinc.org/drugnews/v01/n1702/a06.html. Retrieved 2009-03-29.
- ↑ 30.0 30.1 Rosenberg D (2001-07-02). "Drugs: profits vs. pain relief. Does its maker push Oxycontin too hard?". Newsweek. http://www.newsweek.com/id/78596. Retrieved 2009-04-11.
- ↑ "Editorial: selling drugs legally, but not always safely" (reprint). Roanoke Times. 2001-06-13. http://www.mapinc.org/drugnews/v01/n1052/a08.html. Retrieved 2009-04-11.
- ↑ 32.0 32.1 "Drugmaker to pay $19.5 mil to settle OxyContin lawsuit". Arizona Republic. Associated Press. 2007-05-09. http://www.azcentral.com/arizonarepublic/business/articles/0509biz-oxycontin0509.html. Retrieved 2009-04-11.
- ↑ 33.0 33.1 O'Brien J (2007-05-10). "Purdue pleads out, will pay $634 million in fines". LegalNewsline.com. http://www.legalnewsline.com/news/194919-purdue-pleads-guilty-will-pay-634-million-in-fines. Retrieved 2009-04-11.
- ↑ Chasan E (2007-05-10). "Purdue Frederick pleads guilty in OxyContin case". Reuters. http://www.reuters.com/article/healthNews/idUSWBT00695020070510. Retrieved 2009-04-21.
- ↑ Moore C (2008-06-11). "OxyContin lawsuit stuck in N.Y. court". Appalachian News-Express. http://www.news-expressky.com/articles/2008/06/11/news/01court.txt. Retrieved 2009-04-21.
- ↑ "Search results [for OxyNorm in Pharmaceutical Benefits Schedule"]. Department of Health and Ageing, Australian Government. 2008-06-16. http://www.pbs.gov.au/html/industry/search/results?term=OxyNorm. Retrieved 2009-03-31.
- ↑ "OXYNORM". New Zealand Medicines and Medical Devices Safety Authority. 2008-06-16. http://www.medsafe.govt.nz/Profs/datasheet/o/OxyNormcapsoln.htm. Retrieved 2009-03-31.
- ↑ 38.0 38.1 "Search results [for OxyNorm in electronic Medicines Compendium."]. Datapharm Communications Ltd. http://emc.medicines.org.uk/searchresults.aspx?term=oxynorm&searchtype=QuickSearch. Retrieved 2009-03-31.
- ↑ "OXYNORM injection". New Zealand Medicines and Medical Devices Safety Authority. 2008-06-16. http://www.medsafe.govt.nz/profs/datasheet/o/oxynorminj.htm. Retrieved 2009-03-31.
- ↑ "Percocet (oxycodone and acetaminophen tablets, USP)" (PDF). Endo Pharmaceuticals. 2006. http://www.endo.com/pdf/products/Percocet_pack_insert_2.pdf. Retrieved 2009-03-24.
- ↑ "Ordinanza 16 Giugno 2009" (PDF). Ministero Della Salute. 2009. http://www.sirfarma.it/binary/sirfarma/normativa/Ordinanza_Stupefacenti_Sottosegretario_Fazio_16.06.2009.pdf.
- ↑ "Percodan (oxycodone and aspirin tablets, USP)" (PDF). Endo Pharmaceuticals. 2005. http://www.endo.com/pdf/products/percodan_pack_insert.pdf. Retrieved 2009-03-25.
- ↑ Phebra Pty Ltd (2007-10-19). "Product information Proladone" (PDF). http://www.pharmalab.com.au/resources/products/37/pi/Proladone%20PI%20TAB007%20Version04.pdf. Retrieved 2009-04-09.
- ↑ "Roxicodone" (PDF). Xanodyne Pharmaceuticals Inc. February 2006. http://www.xanodyne.com/pdf/Roxicodone-5mg20mg-orals-tablet.pdf. Retrieved 2009-03-31.
- ↑ "Roxicodone" (PDF). Roxane Laboratories, Inc. http://www.fda.gov/cder/foi/label/2000/21011lbl.pdf. Retrieved 2009-03-31.
- ↑ "Questions and answers for consumers about FDA’s action involving unapproved narcotics containing morphine sulfate, hydromorphone, or oxycodone". U.S. Food and Drug Administration. 2009-03-31. http://www.fda.gov/cder/drug/unapproved_drugs/narcoticsQA.htm. Retrieved 2009-03-31.
- ↑ Riley, J; Eisenberg, E; Müller-Schwefe, G; Drewes, AM; Arendt-Nielsen, L (January 2008). "Oxycodone: a review of its use in the management of pain". Curr Med Res Opin 24 (1): 175–192. doi:10.1185/030079908X253708. ISSN 0300-7995. PMID 18039433. http://www.informapharmascience.com/doi/abs/10.1185/030079908X253708. Retrieved 2009-03-26.
- ↑ Hanks, GW; Conno, F; Cherny, N; Hanna, M; Kalso, E; Mcquay, HJ; Mercadante, S; Meynadier, J et al. (2001-03-02). "Morphine and alternative opioids in cancer pain: the EAPC recommendations." (PDF). Br J Cancer 84 (5): 587–593. doi:10.1054/bjoc.2001.1680. ISSN 0007-0920. PMID 11237376. PMC 2363790. http://www.nature.com/bjc/journal/v84/n5/pdf/6691680a.pdf. Retrieved 2009-03-28.
- ↑ 49.0 49.1 Derek Doyle, etc., (Ed.). (1999). Calman K. Doyle D, Hanks G, Editors. ed. Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press. ISBN 0192625667.
- ↑ Ross FB, Smith MT (November 1997). "The intrinsic antinociceptive effects of oxycodone appear to be κ-opioid receptor mediated". Pain 73 (2): 151–157. doi:10.1016/S0304-3959(97)00093-6. ISSN 0304-3959. PMID 9415500. http://linkinghub.elsevier.com/retrieve/pii/S0304-3959(97)00093-6. Retrieved 2009-04-21.
- ↑ Smith MT (October 2008). "Differences between and combinations of opioids re-visited". Curr Opin Anaesthesiol 21 (5): 596–601. doi:10.1097/ACO.0b013e32830a4c4a. ISSN 0952-7907. PMID 18784485. http://journals.lww.com/co-anesthesiology/pages/articleviewer.aspx?year=2008&issue=10000&article=00013&type=abstract. Retrieved 2009-04-21.
- ↑ Kalso E (December 2007). "How different is oxycodone from morphine?". Pain 132 (3): 227–228. doi:10.1016/j.pain.2007.09.027. ISSN 0304-3959. PMID 17961923. http://linkinghub.elsevier.com/retrieve/pii/S0304-3959(07)00566-0. Retrieved 2009-04-22.
- ↑ Nozaki C, Saitoh A, Kamei J (March 2006). "Characterization of the antinociceptive effects of oxycodone in diabetic mice". Eur. J. Pharmacol. 535 (1-3): 145–151. doi:10.1016/j.ejphar.2006.02.002. ISSN 0014-2999. PMID 16533506. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(06)00128-2. Retrieved 2009-04-22.
- ↑ Nozaki C, Kamei J (April 2007). "Involvement of mu1-opioid receptor on oxycodone-induced antinociception in diabetic mice". Eur. J. Pharmacol. 560 (2-3): 160–162. doi:10.1016/j.ejphar.2007.01.021. ISSN 0014-2999. PMID 17292346. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(07)00038-6. Retrieved 2009-04-22.
- ↑ 55.0 55.1 55.2 Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (May 2006). "Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites" (PDF). Clin Pharmacol Ther 79 (5): 461–479. doi:10.1016/j.clpt.2006.01.009. ISSN 0009-9236. PMID 16678548. http://paincenter.wustl.edu/c/BasicResearch/documents/KharashClinPharm06.pdf. Retrieved 2009-03-28.
- ↑ "''Tentative identification of novel oxycodone metabolites in human urine.'' by Moore KA, Ramcharitar V, Levine B, Fowler D. Office of the Chief Medical Examiner, State of Maryland, 111 Penn Street, Baltimore, Maryland 21201-1020, USA. J Anal Toxicol. 2003 Sep;27(6):346-52". Opioids.com. http://www.opioids.com/oxycodone/metabolites.html. Retrieved 2010-08-01.
- ↑ Isolation and identification of urinary metabolites of oxycodone in rabbits. T Ishida, K Oguri and H Yoshimura.
- ↑ Gasche Y, Daali Y, Fathi M, et al. (December 2004). "Codeine intoxication associated with ultrarapid CYP2D6 metabolism". N Engl J Med 351 (27): 2827–31. doi:10.1056/NEJMoa041888. ISSN 0028-4793. PMID 15625333. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=15625333&promo=ONFLNS19.
- ↑ Otton SV, Wu D, Joffe RT, Cheung SW, Sellers EM (April 1993). "Inhibition by fluoxetine of cytochrome P450 2D6 activity". Clin Pharmacol Ther 53 (4): 401–9. ISSN 0009-9236. PMID 8477556.
- ↑ Analgesic Expert Group. Therapeutic Guidelines: Analgesic. Version 4. Melbourne: Therapeutic Guidelines Ltd, 2007.
- ↑ http://www.pharma.com/PI/Prescription/Oxycontin.pdf
- ↑ Palliative Care Perspectives. James L. Hallenbeck.
- ↑ "Oxycodone Hydrochloride Tablets USP 5 mg, 15 mg, & 30 mg" (PDF). Mallinckrodt Inc. 2007-08-10. http://pharmaceuticals.mallinckrodt.com/_attachments/PackageInserts/57_Oxy%20HCl%20Tabs_REV081007.pdf. Retrieved 2009-03-24.
- ↑ American Society of Health-System Pharmacists (2009-03-23). "Oxycodone". U.S. National Library of Medicine, MedlinePlus. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682132.html. Retrieved 2009-03-27.
- ↑ Oxycodone Side Effects.
- ↑ "Oxycodone Addiction". addictionsearch.com. 2007-02-08. http://www.addictionsearch.com/treatment_articles/article/oxycodone-addiction_14.html.
- ↑ "Oxycodone Professional Monograph - FDA". Drugs.com. http://www.drugs.com/pro/oxycodone.html. Retrieved 2010-08-01.
- ↑ "Commonsense Oxycodone Rx & Safety" (PDF). http://pain-topics.org/pdf/OxycodoneRxSafety.pdf. Retrieved 2010-08-01.
- ↑ "Oxycodone". Center for Substance Abuse Research. 2005-05-02. http://www.cesar.umd.edu/cesar/drugs/oxycodone.asp. Retrieved 2009-03-25.
- ↑ Rao R, Desai NS (June 2002). "OxyContin and neonatal abstinence syndrome" (PDF). J Perinatol 22 (4): 324–5. doi:10.1038/sj.jp.7210744. ISSN 0743-8346. PMID 12032797. http://www.nature.com/jp/journal/v22/n4/pdf/7210744a.pdf. Retrieved 2009-03-25.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1166-1168.
- ↑ "Study finds huge rise in oxycodone deaths". CTV News. http://www.ctv.ca/servlet/ArticleNews/story/CTVNews/20091207/opioids_091207/20091207?hub=TopStoriesV2. Retrieved 2009-12-07.
- ↑ 73.0 73.1 "Action plan to prevent the diversion and abuse of OxyContin". U.S. Drug Enforcement Administration. 2001-06-22. http://www.deadiversion.usdoj.gov/drugs_concern/oxycodone/abuse_oxy.htm. Retrieved 2009-03-31.
- ↑ Tough P (2001-07-29). "The alchemy of OxyContin". New York Times. http://www.nytimes.com/2001/07/29/magazine/the-alchemy-of-oxycontin.html. Retrieved 2009-04-16.
- ↑ Cicero TJ, Inciardi JA, Muñoz A (October 2005). "Trends in abuse of OxyContin and other opioid analgesics in the United States: 2002–2004" (PDF). J Pain 6 (10): 662–72. doi:10.1016/j.jpain.2005.05.004. ISSN 1526-5900. PMID 16202959. http://paincenter.wustl.edu/c/BasicResearch/documents/CiceroJPain2005.pdf.
- ↑ Smith L (2008-06-01). "Panel slams OxyContin trials and says abuse, mortality risks persist". Family Practice News 38 (11): 5. doi:10.1016/S0300-7073(08)70694-7.
- ↑ "OxyContin A Gateway For Young Users In Eastie". WBUR. http://www.wbur.org/2010/04/12/east-boston-oxycontin. Retrieved 2010-08-01.
- ↑ Black E, et al. (2008) (PDF). Australian drug trends 2007. Findings from the Illicit Drug Reporting System (IDRS). Sydney: National Drug and Alcohol Research Centre, University of New South Wales. ISBN 9780733426254. http://ndarc.med.unsw.edu.au/NDARCWeb.nsf/resources/DRUG_TRENDS_1_NAT/$file/DT001.PDF. Retrieved 2009-04-06.
- ↑ Gordon T (2008-03-30). "Scots' use of 'hillbilly heroin' rises by 430%". Sunday Times (London).
- ↑ Thompson T (2002-03-24). "Epidemic fear as 'hillbilly heroin' hits the streets". Society Guardian. http://www.guardian.co.uk/society/2002/mar/24/drugsandalcohol. Retrieved 2009-04-16.
- ↑ Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER (August 7, 2008). "Bidirectional translational research: Progress in understanding addictive diseases.". Neuropharmacology. 56: 32. doi:10.1016/j.neuropharm.2008.07.042. ISSN 0028-3908. PMID 18725235.
- ↑ Rockefeller University (2008-09-10). "Painkiller abuse can predispose adolescents to lifelong addiction". http://newswise.com/articles/view/544184/. Retrieved 2009-03-28.
- ↑ "Summary of Medical Examiner Reports on Oxycodone-Related Deaths". DEA Office of Diversion Control. United States Department of Justice. http://www.deadiversion.usdoj.gov/drugs_concern/oxycodone/oxycontin7.htmÂ. Retrieved 2008-05-18.
- ↑ League of Nations (1931). "Convention for limiting the manufacture and regulating the distribution of narcotic drugs" (PDF). http://treaties.un.org/doc/Treaties/1931/07/19310713%2006-44%20AM/Ch_VI_8_ap.pdf. Retrieved 2009-04-04.
- ↑ 85.0 85.1 "United Nations conference for the adoption of a single convention on narcotic drugs. Final act" (PDF). 1961. http://treaties.un.org/doc/Treaties/1964/12/19641213%2002-14%20AM/Ch_VI_15p.pdf. Retrieved 2009-04-04.
- ↑ Commonwealth of Australia. "Narcotic Drugs Act 1967 - first schedule". Australasian Legal Information Institute. http://www.austlii.edu.au/au/legis/cth/consol_act/nda1967160/sch1.html. Retrieved 2009-04-06.
- ↑ Australian Government. Department of Health and Ageing. Therapeutic Goods Administration (June 2008) (PDF). Standard for the uniform scheduling of drugs and poisons no. 23. Canberra: Commonwealth of Australia. ISBN 1741865964. http://www.comlaw.gov.au/ComLaw/Legislation/LegislativeInstrument1.nsf/0/3BBB39C4645284BCCA2574A6001C711F/$file/PoisonsStandard2008.pdf. Retrieved 2009-04-06.
- ↑ 88.0 88.1 88.2 Canada Department of Justice (2009-02-27). "Controlled Drugs and Substances Act (1996, c. 19)". http://laws.justice.gc.ca/en/ShowFullDoc/cs/C-38.8///en. Retrieved 2009-03-23.
- ↑ 89.0 89.1 German Federal Ministry of Justice (2009-01-19). "Act on the circulation of narcotics (Narcotics Act - BtMG)" (in German). http://bundesrecht.juris.de/btmg_1981/BJNR106810981.html. Retrieved 2009-04-06.
- ↑ 90.0 90.1 90.2 90.3 90.4 Hong Kong Special Administrative Region, People's Republic of China. "Dangerous drugs ordinance - chapter 134". Hong Kong Legal Information Institute. http://www.hklii.org/hk/legis/en/ord/cur/134.txt. Retrieved 2009-04-08.
- ↑ Misuse of Drugs Act (Cap. 185, 2008 Rev. Ed.) (Singapore), section 6(1).
- ↑ Misuse of Drugs Act (Singapore), section 5(1).
- ↑ "List of drugs currently controlled under the Misuse of Drugs legislation". U.K. Home Office. January 2009. http://www.homeoffice.gov.uk/documents/cdlist.pdf?view=Binary. Retrieved 2009-04-08.
- ↑ 94.0 94.1 "Class A, B and C drugs". U.K. Home Office. http://www.homeoffice.gov.uk/drugs/drugs-law/Class-a-b-c/. Retrieved 2009-04-08.
- ↑ "Statutory instrument 2001 No. 3998. The Misuse of Drugs regulations 2001". U.K. Office of Public Sector Information. http://www.opsi.gov.uk/si/si2001/20013998.htm. Retrieved 2009-04-08.
- ↑ 96.0 96.1 96.2 "(United States Code.) Title 21 - food and drugs. Chapter 13 - drug abuse prevention and control" (PDF). U.S. Drug Enforcement Administration. http://www.usdoj.gov/dea/pubs/csa/csa.pdf. Retrieved 2009-04-08.
- ↑ ""FDA Approves New Formulation of OxyContin Designed to reduce abuse of the prescription painkiller", ''consumeraffairs.com'' (April 6, 2010)". Consumeraffairs.com. 2010-04-06. http://www.consumeraffairs.com/news04/2010/04/oxycontin_fda.html. Retrieved 2010-08-01.
- ↑ 98.0 98.1 Babor T, et al., compilers (1994) (PDF). Lexicon of alcohol and drug terms. Geneva: World Health Organization. ISBN 9241544686. http://whqlibdoc.who.int/publications/9241544686.pdf.
Further reading
- Meier, Barry (2003). Pain killer: a "wonder" drug's trail of addiction and death. Emmaus, PA: Rodale. ISBN 1579546382.
- Pinsky, Drew (2004). When painkillers become dangerous: what everyone needs to know about OxyContin and other prescription drugs. Center City, MN: Hazelden. ISBN 159285107X.
- Lockwood, Brad (2006). Oxycontin: from pain relief to addiction. New York: Rosen Pub. Group, Inc. ISBN 9781404209138.
External links
- Coluzzi F, Mattia C. Oxycodone. Pharmacological profile and clinical data in chronic pain management. Minerva Anestesiol 2005 Jul-Aug;71(7-8):451-60.
- Rosenberg T. When is a pain doctor a drug pusher? New York Times 2007 Jun 17.
- Deadly combinations. St. Petersburg Times 2008 Feb 17 - May 19.
- Watch Cottonland, a National Film Board of Canada documentary on OxyContin addiction
- U.S. National Library of Medicine: Drug Information Portal - Oxycodone
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
