Nitroglycerin
| Nitroglycerin | |
|---|---|
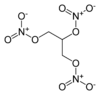 |
 |
|
1,2,3-Trinitroxypropane
|
|
|
2,3-Bis(nitrooxy)propyl nitrate
|
|
|
Other names
1,3-Dinitrooxypropan-2-yl nitrate
Propane-1,2,3-triyl trinitrate |
|
| Identifiers | |
| CAS number | 55-63-0 |
| PubChem | 4510 |
| ChemSpider | 4354 |
| EC number | 200-240-8 |
| UN number | UN 0143, UN 0144, UN 1204, UN 3064, UN 3319 |
| DrugBank | DB00727 |
| KEGG | C07455 |
| MeSH | Nitroglycerin |
| ChEBI | 28787 |
| ATC code | C01,C05AE01 |
| Beilstein Reference | 1802063 |
| Gmelin Reference | 165859 |
|
SMILES
O=N(=O)OCC(CON(=O)=O)ON(=O)=O
|
|
|
InChI
InChI=1S/C3H5N3O9/c7-4(8)13-1-3(15-6(11)12)2-14-5(9)10/h3H,1-2H2
Key: SNIOPGDIGTZGOP-UHFFFAOYSA-N |
|
| Properties | |
| Molecular formula | C3H5N3O9 |
| Molar mass | 227.09 g mol−1 |
| Appearance | Clear yellow/colorless oily liquid |
| Density | 1.6 g/cm³ at 15 °C |
| Melting point |
13.2 °C, 286 K, 56 °F |
| Boiling point |
Decomposes at 50–60 °C (122–140 °F) |
| Explosive data | |
| Shock sensitivity | high |
| Friction sensitivity | high |
| Explosive velocity | 7700 m/s |
| RE factor | 1.50 |
| Hazards | |
| NFPA 704 |
 3
3
4
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Nitroglycerin (NG), (United States spelling) also known as nitroglycerine, (UK spelling), trinitroglycerin, trinitroglycerine, 1,2,3-trinitroxypropane and glyceryl trinitrate, is a heavy, colorless, oily, explosive liquid obtained by nitrating glycerol. Since the 1860s, it has been used as an active ingredient in the manufacture of explosives, specifically dynamite, and as such is employed in the construction and demolition industries. Similarly, since the 1880s, it has been used by the military as an active ingredient, and a gelatinizer for nitrocellulose, in some solid propellants, such as Cordite and Ballistite.
Nitroglycerin is also used medically as a vasodilator to treat heart conditions, such as angina and chronic heart failure. It is one of the oldest and most useful drugs for treating heart disease by shortening or even preventing attacks of angina pectoris. Nitroglycerin comes in forms of tablets, sprays or patches.[1] Nitroglycerin can be used to help destroy prostate cancer[2] as well as being used as a heart medication.
Contents |
History
Nitroglycerin was the first practical explosive stronger than black powder. It was synthesized by chemist Ascanio Sobrero in 1847, working under Théophile-Jules Pelouze at the University of Turin. Sobrero initially called his discovery pyroglycerine, and warned vigorously against its use as an explosive. It was later adopted as a commercially useful explosive by Alfred Nobel. He experimented with safer ways to handle the dangerous substance; his younger brother Emil and several workers were killed in 1864 in a nitroglycerin explosion at the family's armaments factory in Heleneborg, in Sweden.[3]
A year later, Nobel founded Alfred Nobel & Company in Germany, building an isolated factory in the Krümmel hills of Geesthacht near Hamburg. This business exported a liquid combination of nitroglycerin and gunpowder known as "Blasting Oil", but it was extremely unstable and difficult to transport, as shown in numerous catastrophes. The buildings of the Krümmel factory were destroyed twice.[4]
In April 1866, three crates of nitroglycerin were shipped to California for the Central Pacific Railroad, who wished to experiment with its blasting capability to speed the construction of the 1,659-foot (506 m) Summit Tunnel through the Sierra Nevada. One of the crates exploded, destroying a Wells Fargo office in San Francisco and killing 15 people, leading to a complete ban on the transport of liquid nitroglycerin in California. The on-site manufacture of nitroglycerin was thus required for the remaining hard-rock drilling and blasting required for the completion of America's First Transcontinental Railroad.[5]
Liquid nitroglycerin was widely banned elsewhere as well and this finally led to Alfred Nobel & Company developing dynamite in 1867, made by mixing nitroglycerin with the diatomaceous earth (kieselguhr) found in the Krümmel hills. Similar mixtures, such as dualine (1867), lithofracteur (1869), and gelignite (1875), mixed nitroglycerin with other inert absorbents — many combinations were tried to get around Nobel's tightly controlled patents. Dynamites containing nitrocellulose, which increase the viscosity of the mix, are commonly known as "gelatins".
Following discoveries that amyl nitrite helped to alleviate chest pain, Doctor William Murrell experimented with the use of nitroglycerin to alleviate angina pectoris and reduce blood pressure. He began treating patients with small doses in 1878, and it was soon adopted into widespread use after he published his results in The Lancet in 1879. The medical establishment used the name "glyceryl trinitrate" or "trinitrin" to avoid alarming patients who associated nitroglycerin with explosions.[6]
Wartime production rates
Large quantities of nitroglycerin were manufactured in both World Wars for use in military propellants. In World War I HM Factory, Gretna, the largest propellant factory in the United Kingdom, was producing 800 tons (812 tonnes) of Cordite RDB per week. This needed 336 tons of nitroglycerin per week (assuming no losses in production). The Royal Navy had its own factory at Royal Navy Cordite Factory, Holton Heath in Dorset, England. A large cordite factory was built in Canada in World War I. The Canadian Explosives Limited Cordite factory at Nobel, Ontario was designed to produce 1,500,000 lb (680 t) of Cordite per month. It required 286 tonnes of nitroglycerin per month.
Instability and desensitization
In its pure form, it is a primary contact explosive (physical shock can cause it to explode) and degrades over time to even more unstable forms. This makes it highly dangerous to transport or use. In this undiluted form, it is one of the more powerful explosives, comparable to the more recent RDX and PETN, as well as the plastic explosive C-4—which contains over 90% RDX as its active ingredient.
Early in the history of this explosive it was discovered that liquid nitroglycerin can be "desensitized" by cooling to 5 to 10 °C (40 to 50 °F), at which temperature it freezes, contracting upon solidification. However, later thawing can be extremely sensitizing, especially if impurities are present or if warming is too rapid.[7] It is possible to chemically "desensitize" nitroglycerin to a point where it can be considered approximately as "safe" as modern high explosive formulations, by the addition of approximately 10-30% ethanol, acetone,[8] or dinitrotoluene (percentage varies with the desensitizing agent used). Desensitization requires extra effort to reconstitute the "pure" product. Failing this, it must be assumed that desensitized nitroglycerin is substantially more difficult to detonate, possibly rendering it useless as an explosive for practical application.
A serious problem in the use of nitroglycerin results from its high freezing point 13 °C (55 °F). Solid nitroglycerin is much less sensitive to shock than the liquid, a feature common in explosives; in the past it was often shipped in the frozen state, but this resulted in a high number of accidents during the thawing process by the end user just prior to use. This disadvantage is overcome by using mixtures of nitroglycerin with other polynitrates; for example, a mixture of nitroglycerin and ethylene glycol dinitrate freezes at -29 °C (-20 °F).[9]
Detonation
Nitroglycerin and any dilutents can certainly deflagrate, i.e. burn. However, the explosive power of nitroglycerin is derived from detonation: energy from the initial decomposition causes a pressure wave or gradient that detonates the surrounding fuel. This is a self-sustained shock wave that propagates through the explosive medium at some 30 times the speed of sound as a near-instantaneous pressure-induced decomposition of the fuel into a white hot gas. Detonation of nitroglycerin generates gases that would occupy more than 1,200 times the original volume at ordinary room temperature and pressure; moreover, the heat liberated raises the temperature to about 5,000 °C (9,030 °F).[10] This is totally different from deflagration, which depends solely upon available fuel regardless of pressure or shock.
Manufacturing
The industrial manufacturing process often uses a nearly 50:50 mixture of concentrated sulfuric acid and concentrated nitric acid. This can be produced by mixing white fuming nitric acid (quite costly pure nitric acid in which oxides of nitrogen have been removed, as opposed to red fuming nitric acid) and concentrated sulfuric acid. More often, this mixture is attained by the cheaper method of mixing fuming sulfuric acid, also known as oleum, (sulfuric acid containing excess sulfur trioxide) and azeotropic nitric acid (consisting of around 70% nitric acid, the rest being water).
The sulfuric acid produces protonated nitric acid species, which are attacked by glycerin's nucleophilic oxygen atoms. The nitro group is thus added as an ester C-O-NO2 and water is produced. This is different from an aromatic nitration reaction in which nitronium ions are the active species in an electrophilic attack of the molecules' ring system.
The addition of glycerin results in an exothermic reaction (i.e., heat is produced), as usual for mixed acid nitrations. However, if the mixture becomes too hot, it results in runaway, a state of accelerated nitration accompanied by the destructive oxidizing of organic materials of nitric acid and the release of very poisonous brown nitrogen dioxide gas at high risk of an explosion. Thus, the glycerin mixture is added slowly to the reaction vessel containing the mixed acid (not acid to glycerin). The nitrator is cooled with cold water or some other coolant mixture and maintained throughout the glycerin addition at about 22 °C (72 °F), much below which the esterification occurs too slowly to be useful. The nitrator vessel, often constructed of iron or lead and generally stirred with compressed air, has an emergency trap door at its base, which hangs over a large pool of very cold water and into which the whole reaction mixture (called the charge) can be dumped to prevent an explosion, a process referred to as drowning. If the temperature of the charge exceeds about 30 °C (86 °F) (actual value varying by country) or brown fumes are seen in the nitrator's vent, then it is immediately drowned.
Use as an explosive and a propellant

The main use of nitroglycerin, by tonnage, is in explosives such as dynamite and in propellants.
Nitroglycerin is an oil that may explode with heat, pressure or when it burns. It is extremely unstable, therefore dropping or bumping a container can also make it explode.[11]
Alfred Nobel developed the use of nitroglycerin as a blasting explosive by mixing the nitroglycerine with inert absorbents particularly diatomaceous earth. He named this explosive dynamite and patented it in 1867. It was supplied ready for use in the form of sticks, individually wrapped in greased water-proof paper. Dynamite and similar explosives were widely adopted for civil engineering tasks, such as building railway tunnels and cuttings; and for quarrying.
Nitroglycerin was also adapted as a military propellant, for use in guns and rifles. Poudre B, invented in France in 1886, was one of the first military propellants to replace gunpowder; but it was based on nitrocellulose, not nitroglycerin. It was later found to be unstable.
Nitroglycerin is a high explosive which is so unstable that the slightest jolt, friction, or impact can cause it to detonate (the sensitivity, however, is often overestimated; pure samples are quite safe). The molecule contains oxygen, nitrogen, and carbon in weak bonds; when it explodes great energy is released as the atoms rearrange to form new molecules with strong, stable bonds like N2 and CO. It is the speed of the decomposition reaction which makes it such a violent explosive. A supersonic wave passing through the material causes it to decompose almost instantly. This instantaneous destruction of all molecules is called a detonation, and the destructive blast results from the rapid expansion of hot gases. Nitroglycerin has an advantage over some other high explosives, that no visible smoke is produced, therefore acting as a "smokeless powder".[12]
Alfred Nobel then developed ballistite, by combining nitroglycerin and guncotton. He patented it in 1887. Ballistite was adopted by a number of European governments, as a military propellant. Italy was the first to adopt it. However, it was not adopted by the British Government. They, together with the British Commonwealth countries, adopted cordite, which had been developed by Sir Frederick Abel and Sir James Dewar, in 1889. The original Cordite Mk I consisted of 58% nitroglycerine, 37% guncotton and 5% petroleum jelly. Ballistite and cordite were both manufactured in the forms of cords.
Smokeless powders were originally developed using nitrocellulose as the sole explosive ingredient; and were therefore known as single base propellants. A range of smokeless powders that contain both nitrocellulose and nitroglycerin, known as double base propellants, were also developed. Smokeless powders were originally supplied only for military use; however they were also soon developed for civilian use and were quickly adopted for sport. Some are known as sporting powders.
Blasting gelatin, also known as gelignite, was invented by Nobel in 1875, using nitroglycerine, wood pulp, and sodium or potassium nitrates. This was an early low-cost, flexible explosive.
Nitroglycerin and dynamite
Alfred Nobel discovered that mixing nitroglycerin with silica would turn the liquid into a paste, called dynamite. An advantage of dynamite was that it could be cylinder-shaped for insertion into the drilling holes used for mining. Nobel received U.S. patent number 78,317 for his dynamite in 1867.[13]
Medical applications
Medical use
Nitroglycerin belongs to a group of drugs called nitrates, which includes many other nitrates like isosorbide dinitrate (Isordil) and isosorbide mononitrate (Imdur, Ismo, Monoket).[14] In medicine, where it is generally called glyceryl trinitrate, nitroglycerin is used as a heart medication (under the trade names Nitrospan, Nitrostat, and Tridil, amongst others). It is used as a medicine for angina pectoris (ischaemic heart disease) in tablets, ointment, solution for intravenous use, transdermal patches (Trinipatch, Transderm Nitro, Nitro-Dur), or sprays administered sublingually (Nitrolingual Pump Spray, Natispray). Patients who experience angina when doing certain physical activities, can often prevent symptoms by taking nitroglycerin 5 to 10 minutes before the activity also allowing more freedom to enjoy. Some forms of nitroglycerin last much longer in the body than others. These may come in the form of a pill taken one, two, or three times per day, or even as a patch. Proved by research, it is shown that round-the-clock exposure to nitrates can cause the body to stop responding normally to this medicine. Experts recommend that the patches be removed at night, allowing the body a few hours to restore its responsiveness to nitrates. Shorter-acting preparations can be used several times a day with less risk of the body getting used to this drug.[15] Nitroglycerin was synthesized in 1846, and was first used to treat anginal attacks in 1879.
Angina pectoris is due to an inadequate flow of blood and oxygen to the heart which is essential for the production of energy. The heart muscle must produce and use the energy in order to be able to pump blood through the lungs and into the arteries. It is believed that nitroglycerin corrects the imbalance between the flow of oxygen and blood to the heart.[16] The principal action of nitroglycerin is vasodilation—widening of the blood vessels. Nitroglycerin will dilate veins more than arteries because dilation of the veins help so that the heart does less work and requires less oxygen and blood. It also lowers the pressure in the arteries against which the heart must pump.[14] Dilating the veins, decreases cardiac preload and leads to the following therapeutic effects during episodes of angina pectoris:
- subsiding of chest pain
- decrease of blood pressure
- increase of heart rate.
- orthostatic hypotension
These effects arise because nitroglycerin is converted to nitric oxide in the body by mitochondrial aldehyde dehydrogenase,[17] and nitric oxide is a natural vasodilator. Recently, it has also become popular in an off-label use at reduced (0.2%) concentration in ointment form as an effective treatment for anal fissure.
Side effects
The side effects of nitroglycerin include lack of sexual desire, headache, painful urination and increased bowel movements. Patients are often told to sit or lie down during and immediately after taking nitroglycerin to reduce the risk of low blood pressure. A drop in blood pressure can be accompanied by weakness or dizziness.[18]
Shortly after the invention of nitroglycerin, this substance was noticed to be capable of inducing a violent headache. Headaches are the most prominent side effect of nitrate therapy. This was due to the release of nitric oxide (NO) by nitroglycerin. Such studies have led to propose that NO may be the causative molecule in migraine pain. The importance of NO as a potential initiator of the migraine attack opens new directions for other vascular headaches and pharmacological treatment of migraines.[19]
Forms

A common form of medical nitroglycerin is a small white tablet that patients slip under the tongue. Another form is nitroglycerin sprays that are a convenient alternative for someone awakened by angina attacks at night. These two forms are not for routine use, only to be used at the onset of chest pain. A patch is another form of nitroglycerin. The medication contained in the patch is slowly released and absorbed through the skin and into the bloodstream, however it will not relieve an attack that has already started.[20]
Since 1879, nitroglycerin pills have been a standard treatment for angina and heart attacks, but it wasn't until the 1970s that researchers understood that the body converts nitroglycerin into nitric oxide, a messenger molecule that tells the smooth muscles surrounding blood vessels to relax.[21]
When a pill is needed the person places it under the tongue and allows it to dissolve, which usually takes about 20–30 seconds. Nitroglycerin can also be chewed, but is less effective when it is swallowed without being dissolved. Its actions make a gentle tingling sensation under the tongue. Nitroglycerin is more effective when taken at the very inception of chest discomfort. After taking the nitroglycerin pill, relief often follows within one to two minutes, but not all types of chest pain respond to nitroglycerin.[22]
Industrial exposure
Infrequent exposure to high doses of nitroglycerin can cause severe headaches known as "NG head". These headaches can be severe enough to incapacitate some people; however, humans develop a tolerance to and dependence on nitroglycerin after long-term exposure. Withdrawal can (rarely) be fatal; withdrawal symptoms include headaches and heart problems; with re-exposure to nitroglycerin, these symptoms may disappear.
For workers in nitroglycerin (NTG) manufacturing facilities, this can result in a "Monday morning headache" phenomenon for those who experience regular nitroglycerin exposure in the workplace leading to the development of NTG tolerance for the vasodilating effects. Over the weekend the workers lose the tolerance to NTG and when they are reexposed on Monday the prominent vasodilation produces tachycardia, dizziness, and a headache.
See also
- Erythritol tetranitrate
- Ethylene glycol dinitrate
- Mannitol hexanitrate
- Methyl nitrate
- Xylitol pentanitrate
References
- ↑ Feb97, Vol. 7, Issue 6
- ↑ Daily Mail: "How dynamite could help destroy prostate cancer" Retrieved 2010-02-23
- ↑ NobelPrize.org: Emil Nobel.
- ↑ NobelPrize.org: Krümmel.
- ↑ "Transcontinental Railroad - People & Events: Nitroglycerin", American Experience, PBS.
- ↑ Sneader, Walter. Drug Discovery: A History. John Wiley and Sons, 2005 ISBN 0471899801.
- ↑ "Tales of Destruction-Thawing can be Hell". http://www.logwell.com/tales/second_nitro_death.html.
- ↑ "Tales of Destruction-Is Nitroglicerine in This?". http://www.logwell.com/tales/red_glycerin.html.
- ↑ "nitroglycerin". Britannica. http://www.britannica.com/nobel/micro/426_77.html. Retrieved 2005-03-23.
- ↑ Encyclopaedia Britannica.
- ↑ Ch. 3: Explosives and Bombs 1998
- ↑ An explosive combination of atoms
- ↑ About.com
- ↑ 14.0 14.1 Nitroglycerin Article
- ↑ Nitroglycerin for angina, February 1997, Vol. 7.
- ↑ http://www.medicinenet.com/nitroglycerin/article.htm
- ↑ Chen et al., Proc. Natl. Acad. Sci. USA (2005) 102:12159-64.
- ↑ [Nitrohttp://www.medicinenet.com/nitroglycerin/page2.htm Nirtoglycerin Article Page 2]
- ↑ History of nitroglycerin
- ↑ Heart School; Nitroglycerin
- ↑ Nitroglycerin Column
- ↑ The 'mini' Wonder Drug
External links
- "Nitroglycerine! Terrible Explosion and Loss of Lives in San Francisco". Central Pacific Railroad Photographic History Museum. http://CPRR.org/Museum/Newspapers/Nitroglycerine.html. Retrieved 2005-03-23. - 1866 Newspaper article
- WebBook page for C3H5N3O9
- The Tallini Tales of Destruction Detailed and horrific stories of the historical use of nitroglycerin-filled torpedoes to restart petroleum wells.