Neutrophil granulocyte
This article is about neutrophils, cells of the immune system. For organisms that grow in neutral pH environments see: Neutrophile.
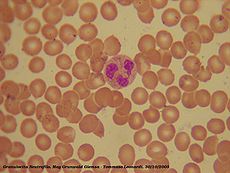
Neutrophil granulocytes are generally referred to as either neutrophils or polymorphonuclear neutrophils (or PMNs), and are subdivided into segmented neutrophils (or segs) and banded neutrophils (or bands). Neutrophils are the most abundant type of white blood cells in mammals and form an essential part of the innate immune system. They form part of the polymorphonuclear cell family (PMNs) together with basophils and eosinophils [1] [2].[3])
The name, neutrophil, derives from staining characteristics on hematoxylin and eosin (H&E) histological or cytological preparations. Whereas basophilic white blood cells stain dark blue and eosinophilic white blood cells stain bright red, neutrophils stain a neutral pink. Normally neutrophils contain a nucleus divided into 2-5 lobes.
Neutrophils are normally found in the blood stream. However, during the beginning (acute) phase of inflammation, particularly as a result of bacterial infection and some cancers[4][5], neutrophils are one of the first-responders of inflammatory cells to migrate toward the site of inflammation, firstly through the blood vessels, then through interstitial tissue, following chemical signals (such as Interleukin-8 (IL-8) and C5a) in a process called chemotaxis. They are the predominant cells in pus, accounting for its whitish/yellowish appearance.
Neutrophils are recruited to the site of injury within minutes following trauma and are the hallmark of acute inflammation.[6]
Contents |
Measurement of neutrophils

Neutrophil granulocytes have an average diameter of 12-15 micrometers (µm) in peripheral blood smears.
With the eosinophil and the basophil, they form the class of polymorphonuclear cells, named for the nucleus's characteristic multilobulated shape (as compared to lymphocytes and monocytes, the other types of white cells). Neutrophils are the most abundant white blood cells in humans (approximately 10^11 are produced daily) ; they account for approximately 70% of all white blood cells (leukocytes).

The stated normal range for human blood counts varies between laboratories, but a neutrophil count of 2.5-7.5 x 109/L is a standard normal range. People of African and Middle Eastern descent may have lower counts, which are still normal.
A report may divide neutrophils into segmented neutrophils and bands.
A minor difference is found between the neutrophils from a male subject and a female subject. The cell nucleus of a neutrophil from a female subject shows a small additional X chromosome structure, known as a "neutrophil drumstick".
Life span
The average half-life of non-activated neutrophils in the circulation is about 12 hours. Upon activation, they marginate (position themselves adjacent to the blood vessel endothelium), and undergo selectin-dependent capture followed by integrin-dependent adhesion in most cases, after which they migrate into tissues, where they survive for 1–2 days.
Neutrophils are much more numerous than the longer-lived monocyte/macrophage phagocytes. A pathogen (disease-causing microorganism or virus) is likely to first encounter a neutrophil. Some experts hypothesize that the short lifetime of neutrophils is an evolutionary adaptation. The short lifetime of neutrophils minimizes propagation of those pathogens that parasitize phagocytes because the more time such parasites spend outside a host cell, the more likely they will be destroyed by some component of the body's defenses. Also, because neutrophil antimicrobial products can also damage host tissues, their short life limits damage to the host during inflammation.
Neutrophils will often be phagocytosed themselves by macrophages after digestion of pathogens. PECAM-1 and phosphatidylserine on the cell surface are involved in this process.
Chemotaxis
Neutrophils undergo a process called chemotaxis, which allows them to migrate toward sites of infection or inflammation. Cell surface receptors allow neutrophils to detect chemical gradients of molecules such as interleukin-8 (IL-8), interferon gamma (IFN-gamma), and C5a, which these cells use to direct the path of their migration.
Anti-microbial function

Being highly motile, neutrophils quickly congregate at a focus of infection, attracted by cytokines expressed by activated endothelium, mast cells, and macrophages. Neutrophils express[7] and release cytokines, which in turn amplify inflammatory reactions by several other cell types.
In addition to recruiting and activating other cells of the immune system, neutrophils play a key role in the front-line defence against invading pathogens. Neutrophils have three strategies for directly attacking micro-organisms: phagocytosis (ingestion), release of soluble anti-microbials (including granule proteins) and generation of neutrophil extracellular traps (NETs) [8].
Phagocytosis
Neutrophils are phagocytes, capable of ingesting microorganisms or particles. They can internalize and kill many microbes, each phagocytic event resulting in the formation of a phagosome into which reactive oxygen species and hydrolytic enzymes are secreted. The consumption of oxygen during the generation of reactive oxygen species has been termed the "respiratory burst", although unrelated to respiration or energy production.
The respiratory burst involves the activation of the enzyme NADPH oxidase, which produces large quantities of superoxide, a reactive oxygen species. Superoxide dismutates, spontaneously or through catalysis via enzymes known as superoxide dismutases (Cu/ZnSOD and MnSOD), to hydrogen peroxide, which is then converted to hypochlorous acid HClO, by the green heme enzyme myeloperoxidase. It is thought that the bactericidal properties of HClO are enough to kill bacteria phagocytosed by the neutrophil, but this may instead be step necessary for the activation of proteases.[9].
Degranulation
Neutrophils also release an assortment of proteins in three types of granules by a process called degranulation:
| Granule type | Protein |
| specific granules (or "secondary granules") | Lactoferrin and Cathelicidin |
| azurophilic granules (or "primary granules") | myeloperoxidase, bactericidal/permeability increasing protein (BPI), Defensins and the serine proteases neutrophil elastase and cathepsin G |
| tertiary granules | cathepsin and gelatinase |
Neutrophil Extracellular Traps(NETs)
Zychlinsky and colleagues recently described a new striking observation that activation of neutrophils causes the release of web-like structures of DNA; this represents a third mechanism for killing bacteria.[10] These neutrophil extracellular traps (NETs) comprise a web of fibers composed of chromatin and serine proteases that trap and kill microbes extracellularly. It is suggested that NETs provide a high local concentration of antimicrobial components and bind, disarm, and kill microbes independent of phagocytic uptake. In addition to their possible antimicrobial properties, NETs may serve as a physical barrier that prevents further spread of pathogens. Trapping of bacteria may be a particularly important role for NETs in sepsis, where NET are formed within blood vessels.[11] Recently, NETs have been shown to play a role in inflammatory diseases, as NETs could be detected in preeclampsia, a pregnancy related inflammatory disorder in which neutrophils are known to be activated.[12]
Role in disease
Low neutrophil counts are termed neutropenia. This can be congenital (genetic disorder) or it can develop later, as in the case of aplastic anemia or some kinds of leukemia. It can also be a side-effect of medication, most prominently chemotherapy. Neutropenia makes an individual highly susceptible to infections. Neutropenia can be the result of colonization by intracellular neutrophilic parasites.
Functional disorders of neutrophils are often hereditary. They are disorders of phagocytosis or deficiencies in the respiratory burst (as in chronic granulomatous disease, a rare immune deficiency, and myeloperoxidase deficiency).
In alpha 1-antitrypsin deficiency, the important neutrophil enzyme elastase is not adequately inhibited by alpha 1-antitrypsin, leading to excessive tissue damage in the presence of inflammation - most prominently pulmonary emphysema.
In Familial Mediterranean fever (FMF), a mutation in the pyrin (or marenostrin) gene, which is expressed mainly in neutrophil granulocytes, leads to a constitutively active acute phase response and causes attacks of fever, arthralgia, peritonitis, and - eventually - amyloidosis.[13]
Media
[1] Neutrophils display highly directional amoeboid motility in infected footpad and phalanges. Intravital imaging was performed in the footpad path of LysM-eGFP mice 20 min after infection with LM.[14] Public Library of Science
Additional images
 A scanning electron microscope image of a single neutrophil (yellow), engulfing anthrax bacteria (orange) |
 Blood cell lineage |
_diagram.png) More complete lineages (very large) |
References
- ↑ Witko-Sarsat, V; Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L (2000). "Neutrophils: molecules, functions and pathophysiological aspects". Lab Invest 80 (5): 617–53. PMID 10830774. http://www.nature.com/labinvest/journal/v80/n5/full/3780067a.html.
- ↑ Klebanoff, SJ; Clark, RA (1978). The Neutrophil: Function and Clinical Disorders. Elsevier/North-Holland Amsterdam. ISBN 0444800204.
- ↑ Nathan, C (2006). "Neutrophils and immunity: challenges and opportunities". Nature Reviews Immunology 6 (March): 173–82. doi:10.1038/nri1785. ISSN 1474-1733. PMID 16498448. http://www.nature.com/nri/journal/v6/n3/abs/nri1785.html.
- ↑ Waugh, DJ; Wilson (2008). "The interleukin-8 pathway in cancer.". Clinical Cancer Research 14 (21): 6735–41. doi:10.1158/1078-0432.CCR-07-4843. ISSN 1078-0432. PMID 18980965. http://clincancerres.aacrjournals.org/cgi/content/abstract/14/21/6735.
- ↑ De Larco, JE; Wuertz; Furcht (2004). "The Potential Role of Neutrophils in Promoting the Metastatic Phenotype of Tumors Releasing Interleukin-8.". Clinical Cancer Research 10 (15): 4895–900. doi:10.1158/1078-0432.CCR-03-0760. ISSN 1078-0432. PMID 15297389. http://clincancerres.aacrjournals.org/cgi/content/full/10/15/4895.
- ↑ Cohen, Stephen. Burns, Richard C. Pathways of the Pulp, 8th Edition. St. Louis: Mosby, Inc. 2002. page 465.
- ↑ Ear T, McDonald PP. Cytokine generation, promoter activation, and oxidant-independent NF-kappaB activation in a transfectable human neutrophilic cellular model. BMC Immunol. 2008 Apr 11;9:14. PMID: 18405381
- ↑ Hickey, MJ; Kubes P (2009). "Intravascular immunity: the host–pathogen encounter in blood vessels". Nature Reviews Immunology (Nature Publishing Group) 9 ((5)): 364–75. doi:10.1038/nri2532. PMID 19390567.
- ↑ Segal, AW (2005). "How neutrophils kill microbes.". Annu Rev Immunol 9 ((5)): 197–223. doi:10.1146/annurev.immunol.23.021704.115653. PMID 15771570. PMC 2092448. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2092448/?tool=pubmed.
- ↑ Brinkmann, Volker; Ulrike Reichard, Christian Goosmann, Beatrix Fauler, Yvonne Uhlemann, David S. Weiss, Yvette Weinrauch, Arturo Zychlinsky (5 March 2004). "Neutrophil Extracellular Traps Kill Bacteria" (HTML, PDF). Science (AAAS) 303 (5663): 1532–1535. doi:10.1126/science.1092385. ISSN 0036-8075. PMID 15001782. http://www.sciencemag.org/cgi/content/full/303/5663/1532. Retrieved 2007-04-09.
- ↑ Clark, SR; Ma, AC; Tavener, SA; Mcdonald, B; Goodarzi, Z; Kelly, MM; Patel, KD; Chakrabarti, S et al.; Ma AC, Tavener AS, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, DeVinney R, Doig CJ, Green FHY and Kubes P (2007). "Platelet Toll-Like Receptor-4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Endotoxemic and Septic Blood". Nature Medicine (Nature Publishing Group) 13 ((4)): 463–9. doi:10.1038/nm1565. ISSN 1078-8956. PMID 17384648. http://www.nature.com/nm/journal/v13/n4/pdf/nm1565.pdf.
- ↑ Gupta, AK; Hasler, P; Holzgreve, W; Hahn, S; Hasler P, Holzgreve W, Hahn S. (2007). "Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia?". Semin Immunopathol 29 (2): 163–7. doi:10.1007/s00281-007-0073-4. ISSN 1863-2297. PMID 17621701.
- ↑ Ozen, S (2004). "Familial mediterranean fever: revisiting an ancient disease.". European Journal of Pediatrics 162 (7-8): 449–54. doi:10.1007/s00431-003-1223-x. ISSN 0340-6199. PMID 12751000. http://www.springerlink.com/content/58qkf57xwkb58enf.
- ↑ Graham, D.B., B.H. Zinselmeyer, F. Mascarenhas, R. Delgado, M.J. Miller, and W. Swat. 2009. ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo. PLoS ONE 4:e4652.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||