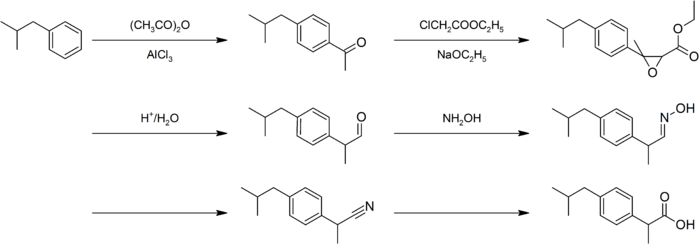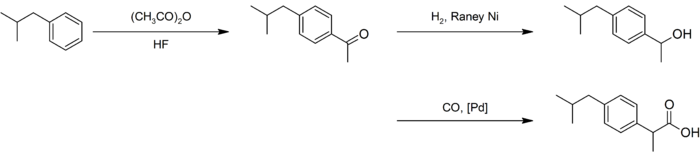Ibuprofen
 |
|
|---|---|
| Systematic (IUPAC) name | |
| (RS)-2-(4-(2-methylpropyl)phenyl)propanoic acid | |
| Identifiers | |
| CAS number | 15687-27-1 |
| ATC code | M01AE01 |
| PubChem | CID 3672 |
| DrugBank | DB01050 |
| ChemSpider | 3544 |
| Chemical data | |
| Formula | C13H18O2 |
| Mol. mass | 206.28 |
| SMILES | eMolecules & PubChem |
| Physical data | |
| Melt. point | 76 °C (169 °F) |
| Pharmacokinetic data | |
| Bioavailability | 49–73% |
| Protein binding | 99% |
| Metabolism | Hepatic (CYP2C9) |
| Half-life | 1.8–2 h |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data | US FDA:link |
| Pregnancy cat. | C(AU) D(US) |
| Legal status | Unscheduled (AU) GSL (UK) OTC (US) |
| Routes | Oral, rectal, topical, and intravenous |
| |
|

Ibuprofen (INN) (pronounced /ˈaɪbjuːproʊfɛn/ or /aɪbjuːˈproʊfən/; from the now-outdated nomenclature iso-butyl-propanoic-phenolic acid) is a non-steroidal anti-inflammatory drug (NSAID) originally marketed as Brufen, and since then under various other trademarks (see tradenames section), most notably Nurofen, Advil, and Motrin. It is used for relief of symptoms of arthritis, primary dysmenorrhea, fever, and as an analgesic, especially where there is an inflammatory component. Ibuprofen is known to have an antiplatelet effect, though it is relatively mild and short-lived when compared with that of aspirin or other better-known antiplatelet drugs. Ibuprofen also generally acts as a vasodilator, having been shown to dilate coronary arteries and some other blood vessels. Ibuprofen is a core medicine in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system.[1]
Contents |
History
Ibuprofen was derived from propionic acid by the research arm of Boots Group during the 1960s.[2] It was discovered by Andrew RM Dunlop, with colleagues Stewart Adams, John Nicholson, Jeff Wilson & Colin Burrows and was patented in 1961. The drug was launched as a treatment for rheumatoid arthritis in the United Kingdom in 1969, and in the United States in 1974. Dr. Adams initially tested his drug on a hangover. He was subsequently awarded an OBE in 1987. Boots was awarded the Queen's Award For Technical Achievement for the development of the drug in 1987.[3]
Typical administration
Low doses of ibuprofen (200 mg, and sometimes 400 mg) are available over the counter (OTC) in most countries. Ibuprofen has a dose-dependent duration of action of approximately 4–8 hours, which is longer than suggested by its short half-life. The recommended dose varies with body mass and indication. Generally, the oral dose is 200–400 mg (5–10 mg/kg in children) every 4–6 hours, adding up to a usual daily dose of 800–1,200 mg. 1,200 mg is considered the maximum daily dose for over-the-counter use, though under medical direction, the maximum amount of ibuprofen for adults is 800 milligrams per dose or 3200 mg per day (4 maximum doses).
Unlike aspirin, which breaks down in solution, ibuprofen is stable, and thus ibuprofen can be available in topical gel form which is absorbed through the skin, and can be used for sports injuries, with less risk of digestive problems.[4]
Off-label and investigational use
Ibuprofen is sometimes used for the treatment of acne, because of its anti-inflammatory properties,[5] and has been sold in Japan in topical form for adult acne.[6]
As with other NSAIDs, ibuprofen may be useful in the treatment of severe orthostatic hypotension (low blood pressure when standing up).[7]
In some studies, ibuprofen showed superior results compared to a placebo in the prophylaxis of Alzheimer's disease, when given in low doses over a long time.[8] Further studies are needed to confirm the results before ibuprofen can be recommended for this indication.
Ibuprofen has been associated with a lower risk of Parkinson's disease, and may delay or prevent it. Aspirin, other NSAIDs, and paracetamol (acetaminophen) had no effect on the risk for Parkinson's.[9] Further research is warranted before recommending ibuprofen for this use.
Ibuprofen lysine
In Europe, Australia, and New Zealand, ibuprofen lysine (the lysine salt of ibuprofen, sometimes called "ibuprofen lysinate" even though the lysine is in cationic form) is licensed for treatment of the same conditions as ibuprofen. The lysine salt increases water solubility, allowing the medication to be administered intravenously.[10] Ibuprofen lysine has been shown to have a more rapid onset of action compared to acid ibuprofen.[11]
Ibuprofen lysine is indicated for closure of a patent ductus arteriosus in premature infants weighing between 500 and 1500 grams, who are no more than 32 weeks gestational age when usual medical management (e.g., fluid restriction, diuretics, respiratory support, etc.) is ineffective.[10] With regard to this indication, ibuprofen lysine is an effective alternative to intravenous indomethacin and may be advantageous in terms of renal function.[12]
Mechanism of action
Non-steroidal anti-inflammatory drugs such as ibuprofen work by inhibiting the enzyme cyclooxygenase (COX), which converts arachidonic acid to prostaglandin H2 (PGH2). PGH2, in turn, is converted by other enzymes to several other prostaglandins (which are mediators of pain, inflammation, and fever) and to thromboxane A2 (which stimulates platelet aggregation, leading to the formation of blood clots).
Like aspirin, indomethacin, and most other NSAIDs, ibuprofen is considered a non-selective COX inhibitor—that is, it inhibits two isoforms of cyclooxygenase, COX-1 and COX-2. The analgesic, antipyretic, and anti-inflammatory activity of NSAIDs appears to be achieved mainly through inhibition of COX-2, whereas inhibition of COX-1 would be responsible for unwanted effects on platelet aggregation and the gastrointestinal tract.[13] However, the role of the individual COX isoforms in the analgesic, anti-inflammatory, and gastric damage effects of NSAIDs is uncertain and different compounds cause different degrees of analgesia and gastric damage.[14] In order to achieve the beneficial effects of ibuprofen and other NSAIDS without gastrointestinal uleceration and bleeding, selective COX-2 inhibitors were developed to inhibit the COX-2 isoform without inhibition of COX-1.[15]
Adverse effects
Ibuprofen appears to have the lowest incidence of digestive adverse drug reactions (ADRs) of all the non-selective NSAIDs. However, this only holds true at lower doses of ibuprofen, so over-the-counter preparations of ibuprofen are generally labeled to advise a maximum daily dose of 1,200 mg.[16][17]
Common adverse effects include: nausea, dyspepsia, gastrointestinal ulceration/bleeding, raised liver enzymes, diarrhea, constipation, epistaxis, headache, dizziness, priapism, rash, salt and fluid retention, and hypertension.[18]. A study from 2010 has shown that regular use of NSAIDs was associated with an increase in hearing loss.[19]
Infrequent adverse effects include: esophageal ulceration, heart failure, hyperkalemia, renal impairment, confusion, and bronchospasm.[18]
Photosensitivity
As with other NSAIDs, ibuprofen has been reported to be a photosensitising agent.[20][21] However, this only rarely occurs with ibuprofen and it is considered to be a very weak photosensitising agent when compared with other members of the 2-arylpropionic acid class. This is because the ibuprofen molecule contains only a single phenyl moiety and no bond conjugation, resulting in a very weak chromophore system and a very weak absorption spectrum which does not reach into the solar spectrum.
Cardiovascular risk
Along with several other NSAIDs, ibuprofen has been implicated in elevating the risk of myocardial infarction (heart attack), particularly among those chronically using high doses.[22]
Risks in inflammatory bowel disease (IBD)
Ibuprofen should not be used regularly in individuals with inflammatory bowel disease due to its ability to cause gastric bleeding and form ulceration in the gastric lining. Pain relievers such as paracetemol/acetaminophen or drugs containing codeine (which slows down bowel activity) are safer methods than ibuprofen for pain relief from IBD. Ibuprofen is also known to cause worsening of IBD during flare-ups, so it should be avoided completely at those times.
Human toxicology
Ibuprofen overdose has become common since it was licensed for over-the-counter use. There are many overdose experiences reported in the medical literature, although the frequency of life-threatening complications from ibuprofen overdose is low.[23] Human response in cases of overdose ranges from absence of symptoms to fatal outcome in spite of intensive care treatment. Most symptoms are an excess of the pharmacological action of ibuprofen and include abdominal pain, nausea, vomiting, drowsiness, dizziness, headache, tinnitus, and nystagmus. Rarely more severe symptoms such as gastrointestinal bleeding, seizures, metabolic acidosis, hyperkalaemia, hypotension, bradycardia, tachycardia, atrial fibrillation, coma, hepatic dysfunction, acute renal failure, cyanosis, respiratory depression, and cardiac arrest have been reported.[24] The severity of symptoms varies with the ingested dose and the time elapsed; however, individual sensitivity also plays an important role. Generally, the symptoms observed with an overdose of ibuprofen are similar to the symptoms caused by overdoses of other NSAIDs.
There is little correlation between severity of symptoms and measured ibuprofen plasma levels. Toxic effects are unlikely at doses below 100 mg/kg but can be severe above 400 mg/kg; (around 150 200 mg tablets for an average man)[25] however, large doses do not indicate that the clinical course is likely to be lethal.[26] It is not possible to determine a precise lethal dose, as this may vary with age, weight, and concomitant diseases of the individual patient.
Therapy is largely symptomatic. In cases presenting early, gastric decontamination is recommended. This is achieved using activated charcoal; charcoal absorbs the drug before it can enter the systemic circulation. Gastric lavage is now rarely used, but can be considered if the amount ingested is potentially life threatening and it can be performed within 60 minutes of ingestion. Emesis is not recommended.[27] The majority of ibuprofen ingestions produce only mild effects and the management of overdose is straightforward. Standard measures to maintain normal urine output should be instituted and renal function monitored.[25] Since ibuprofen has acidic properties and is also excreted in the urine, forced alkaline diuresis is theoretically beneficial. However, because ibuprofen is highly protein bound in the blood, there is minimal renal excretion of unchanged drug. Forced alkaline diuresis is therefore of limited benefit.[28] Symptomatic therapy for hypotension, GI bleeding, acidosis, and renal toxicity may be indicated. Occasionally, close monitoring in an intensive care unit for several days is necessary. If a patient survives the acute intoxication, they will usually experience no late sequelae.
Detection in body fluids
Ibuprofen may be quantitated in blood, plasma or serum to demonstrate the presence of the drug in a person who has experienced an anaphylactic reaction, confirm a diagnosis of poisoning in hospitalized patients or to assist in a medicolegal death investigation. A nomogram has been published that relates the ibuprofen plasma concentration, time since ingestion and risk of developing renal toxicity in overdose patients.[29]
Chemistry
Ibuprofen is only very slightly soluble in water. Less than 1 mg of ibuprofen dissolves in 1 ml water (< 1 mg/mL).[30] However, it is much more soluble in alcohol/water mixtures.
Stereochemistry
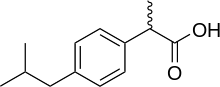
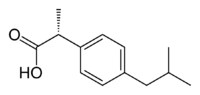
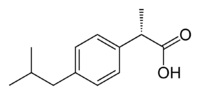
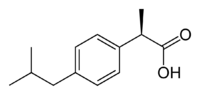
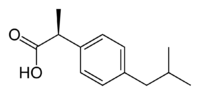
Ibuprofen, like other 2-arylpropionate derivatives (including ketoprofen, flurbiprofen, naproxen, etc), contains a stereocenter in the α-position of the propionate moiety. As such, there are two possible enantiomers of ibuprofen, with the potential for different biological effects and metabolism for each enantiomer.
Indeed it was found that (S)-(+)-ibuprofen (dexibuprofen) was the active form both in vitro and in vivo.
It was logical, then, that there was the potential for improving the selectivity and potency of ibuprofen formulations by marketing ibuprofen as a single-enantiomer product (as occurs with naproxen, another NSAID).
Further in vivo testing, however, revealed the existence of an isomerase (2-arylpropionyl-CoA epimerase) which converted (R)-ibuprofen to the active (S)-enantiomer.[31][32][33] Due to the expense and probable futility of making pure (S)-(+)-ibuprofen, most ibuprofen formulations currently marketed are racemic mixtures.
 |
 |
 |
 |
 |
-ibuprofen-3D-balls.png) |
|
|
|
Synthesis
The synthesis of this compound is a popular case study in green chemistry. The original Boots synthesis of ibuprofen consisted of six steps, started with the Friedel-Crafts acetylation of isobutylbenzene. Reaction with ethyl chloroacetate (Darzens reaction) gave the α,β-epoxy ester, which was decarboxylated and hydrolyzed to the aldehyde. Reaction with hydroxylamine gave the oxime, converted to the nitrile, then hydrolyzed to the desired acid:[34]
An improved synthesis by BHC required only three steps. This improved synthesis won the Presidential Green Chemistry Challenge Greener Synthetic Pathways Award in 1997.[35] After a similar acetylation, hydrogenation with Raney nickel gave the alcohol, which underwent palladium-catalyzed carbonylation:[34]
Availability
Ibuprofen was made available under prescription in the United Kingdom in 1969, and in the United States in 1974. In the years since, the good tolerability profile along with extensive experience in the population, as well as in so-called Phase IV trials (post-approval studies), has resulted in the availability of small packages of ibuprofen over the counter in pharmacies worldwide, as well as in supermarkets and other general retailers.
North America
In the United States, the Food and Drug Administration approved ibuprofen in 200 mg doses for over the counter use in 1984, and is commonly available. Higher doses are only available in North America by prescription.
In 2009, the first injectable formulation of ibuprofen was approved in the United States, under the trade name Caldolor. Ibuprofen thus became the only parenteral for both pain and fever available in the country.[36]
Europe
For some time, there has been a limit on the amount that can be bought over the counter in a single transaction in the UK. Behind the counter in pharmacies this is one pack of 96 × 200 mg or 400 mg, the latter being far less common for over the counter sales. In UK non-pharmacy outlets only 200 mg tablets are allowed and they are restricted to a maximum pack of 16 tablets.
In Germany, 600 mg and 800 mg per pill packages have to be prescribed, whereas 400 mg is available over the counter in pharmacies. In Italy, Belgium and the Netherlands, 200 mg and 400 mg pills are available with no prescription.
In other countries, higher dosages of 600 mg are available.
References
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. March 2005. http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. Retrieved 2006-03-12.
- ↑ Adams SS (April 1992). "The propionic acids: a personal perspective". J Clin Pharmacol 32 (4): 317–23. PMID 1569234. http://jcp.sagepub.com/cgi/reprint/32/4/317.
- ↑ Lambert, Victoria (2007-10-08). "Dr Stewart Adams: 'I tested ibuprofen on my hangover' - Telegraph". The Daily Telegraph (London). http://www.telegraph.co.uk/health/main.jhtml?xml=/health/2007/10/08/hadams108.xml. Retrieved 2008-01-20.
- ↑ "Topical NSAIDs: plasma and tissue concentrations". Bandolier. http://www.medicine.ox.ac.uk/bandolier/booth/painpag/topical/topkin.html.
- ↑ Wong RC, Kang S, Heezen JL, Voorhees JJ, Ellis CN (December 1984). "Oral ibuprofen and tetracycline for the treatment of acne vulgaris". J. Am. Acad. Dermatol. 11 (6): 1076–81. doi:10.1016/S0190-9622(84)80192-9. PMID 6239884.
- ↑ "In Japan, an OTC ibuprofen ointment (Fukidia) for alleviating adult acne has been launched". Inpharma 1 (1530): 18. March 25, 2006. http://www.ingentaconnect.com/content/adis/inp/2006/00000001/00001530/art00048;jsessionid=1ghdlu0vup2pl.alice.
- ↑ Zawada, E. (1982). "Renal consequences of nonsteroidal antiinflammatory drugs". Postgrad Med 71 (5): 223–230. PMID 7041104.
- ↑ Townsend KP, Praticò D (October 2005). "Novel therapeutic opportunities for Alzheimer's disease: focus on nonsteroidal anti-inflammatory drugs". FASEB J. 19 (12): 1592–601. doi:10.1096/fj.04-3620rev. PMID 16195368. http://www.fasebj.org/cgi/content/full/19/12/1592.
- ↑ Chen, H.; Jacobs, E.; Schwarzschild, M.; McCullough, M.; Calle, E.; Thun, M.; Ascherio, A. (2005). "Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease". Ann Neurol 58 (6): 963–7. doi:10.1002/ana.20682. PMID 16240369.
- ↑ 10.0 10.1 Ovation Pharmaceuticals. "Neoprofen (ibuprofen lysine) injection". Package insert.
- ↑ Geisslinger G, Dietzel K, Bezler H, Nuernberg B, Brune K (1989). "Therapeutically relevant differences in the pharmacokinetical and pharmaceutical behavior of ibuprofen lysinate as compared with ibuprofen acid.". Int J Clin Pharmacol Ther Toxicol 27 (7): 324–8. PMID 2777420.
- ↑ Su PH, Chen JY, Su CM, Huang TC, Lee HS (2003). "Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants". Pediatr Int 45 (6): 665–70. doi:10.1111/j.1442-200X.2003.01797.x. PMID 14651538.
- ↑ Rao P, Knaus EE (2008). "Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond". J Pharm Pharm Sci 11 (2): 81s–110s. PMID 19203472. https://ejournals.library.ualberta.ca/index.php/JPPS/article/viewFile/4128/3358.
- ↑ Kakuta H, Zheng X, Oda H, et al. (April 2008). "Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1 selective inhibitor". J. Med. Chem. 51 (8): 2400–11. doi:10.1021/jm701191z. PMID 18363350.
- ↑ "Pain Medications". eMedicine. 2006-02-13. http://www.imedicine.com/DisplayTopic.asp?bookid=99&topic=12021. Retrieved 2010-06-07.
- ↑ "Ibuprofen - Drug information". Medic8.com. http://www.medic8.com/medicines/Ibuprofen.html.
- ↑ "Ibuprofen - Adverse effects". Global Oneness. http://www.experiencefestival.com/a/Ibuprofen_-_Adverse_effects/id/1494737.
- ↑ 18.0 18.1 Rossi S, ed (2004). Australian Medicines Handbook (2004 ed.). Australian Medicines Handbook. ISBN 0-9578521-4-2. OCLC 224121065.
- ↑ Curhan SG, Eavey R, Shargorodsky J, Curhan GC (March 2010). "Analgesic use and the risk of hearing loss in men". Am. J. Med. 123 (3): 231–7. doi:10.1016/j.amjmed.2009.08.006. PMID 20193831. PMC 2831770. http://www.amjmed.com/article/S0002-9343%2809%2900795-5/abstract.
- ↑ Bergner T, Przybilla B (January 1992). "Photosensitization caused by ibuprofen". J. Am. Acad. Dermatol. 26 (1): 114–6. doi:10.1016/0190-9622(92)70018-B. PMID 1531054.
- ↑ Thomson Healthcare. USP DI Advice for the Patient: Anti-inflammatory Drugs, Nonsteroidal (Systemic) [monograph on the internet]. Bethesda (MD): U.S. National Library of Medicine; c2006 [updated 2006 Jul 28; cited 2006 Aug 5]. MedlinePlus DrugInfo uspdi-202743
- ↑ Hippisley-Cox J, Coupland C (2005). "Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis.". BMJ 330 (7504): 1366. doi:10.1136/bmj.330.7504.1366. PMID 15947398. PMC 558288. http://bmj.bmjjournals.com/cgi/content/full/330/7504/1366.
- ↑ McElwee NE, Veltri JC, Bradford DC, Rollins DE. (1990). "A prospective, population-based study of acute ibuprofen overdose: complications are rare and routine serum levels not warranted.". Ann Emerg Med 19 (6): 657–62. doi:10.1016/S0196-0644(05)82471-0. PMID 2188537.
- ↑ Vale JA, Meredith TJ. (1986). "Acute poisoning due to non-steroidal anti-inflammatory drugs. Clinical features and management.". Med Toxicol 1 (1): 12–31. PMID 3537613.
- ↑ 25.0 25.1 Volans G, Hartley V, McCrea S, Monaghan J. (2003). "Non-opioid analgesic poisoning". Clinical Medicine 3 (2): 119–23. doi:10.1007/s10238-003-0014-z. PMID 12737366.
- ↑ Seifert SA, Bronstein AC, McGuire T (2000). "Massive ibuprofen ingestion with survival". J. Toxicol. Clin. Toxicol. 38 (1): 55–7. doi:10.1081/CLT-100100917. PMID 10696926.
- ↑ American Academy Of Clinical Toxico; European Association Of Poisons Cen (2004). "Position paper: Ipecac syrup". J. Toxicol. Clin. Toxicol. 42 (2): 133–43. doi:10.1081/CLT-120037421. PMID 15214617.
- ↑ Hall AH, Smolinske SC, Conrad FL, et al. (1986). "Ibuprofen overdose: 126 cases". Ann Emerg Med 15 (11): 1308–13. doi:10.1016/S0196-0644(86)80617-5. PMID 3777588.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 758-761.
- ↑ Motrin (Ibuprofen) drug description - FDA approved labeling for prescription drugs and medications at RxList
- ↑ Chen CS, Shieh WR, Lu PH, Harriman S, Chen CY (1991). "Metabolic stereoisomeric inversion of ibuprofen in mammals". Biochim Biophys Acta 1078 (3): 411–7. PMID 1859831.
- ↑ Tracy TS, Hall SD (1992). "Metabolic inversion of (R)-ibuprofen. Epimerization and hydrolysis of ibuprofenyl-coenzyme A". Drug Metab Dispos 20 (2): 322–7. PMID 1352228.
- ↑ Reichel C, Brugger R, Bang H, Geisslinger G, Brune K (1997). "Molecular cloning and expression of a 2-arylpropionyl-coenzyme A epimerase: a key enzyme in the inversion metabolism of ibuprofen". Mol Pharmacol 51 (4): 576–82. PMID 9106621. http://molpharm.aspetjournals.org/cgi/content/full/51/4/576.
- ↑ 34.0 34.1 http://www.rsc.org/education/teachers/learnnet/green/ibuprofen/
- ↑ "Presidential Green Chemistry Challenge: 1997 Greener Synthetic Pathways Award". U.S. Environmental Protection Agency. http://www.epa.gov/greenchemistry/pubs/pgcc/winners/gspa97.html. Retrieved 2009-08-18.
- ↑ Drugs.com (June 11, 2009). "FDA Approves Caldolor: Cumberland Pharmaceuticals Announces FDA Approval of Caldolor". Press release. http://www.drugs.com/newdrugs/cumberland-pharmaceuticals-announces-fda-approval-caldolor-1447.html. Retrieved 2009-06-13.
External links
- U.S. National Library of Medicine: MedlinePlus Drug Information: Ibuprofen
- University of Bristol chemistry department page on Ibuprofen
- Nurofen UK Website
- U.S. National Library of Medicine: Drug Information Portal - Ibuprofen
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
|||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||