Molecular mass
The molecular mass (m) of a substance is the mass of one molecule of that substance, in unified atomic mass unit(s) u[1] (equal to 1/12 the mass of one atom of the isotope carbon-12[2]). This is numerically equivalent to the relative molecular mass (Mr) of a molecule, frequently referred to by the term molecular weight, which is the ratio of the mass of that molecule to 1/12 of the mass of carbon-12 and is a dimensionless number. Thus, it is incorrect to express relative molecular mass (molecular weight) in daltons (Da). Unfortunately, the terms molecular weight and molecular mass have been confused on numerous websites, which often state that molecular weight was used in the past as another term for molecular mass.
Molecular mass differs from more common measurements of the mass of chemicals, such as molar mass, by taking into account the isotopic composition of a molecule rather than the average isotopic distribution of many molecules. As a result, molecular mass is a more precise number than molar mass; however it is more accurate to use molar mass on bulk samples. This means that molar mass is appropriate most of the time except when dealing with single molecules.
The concept is important for all molecules, especially for complex molecules like polymers and biopolymers such as proteins and carbohydrates. The determination of their molecular mass is often difficult, and is usually inferred from gel permeation chromatography and mass spectroscopy.
Contents |
Definition
There are varying interpretations of this definition. Many chemists use molecular mass as a synonym of molar mass,[3] differing only in units (see average molecular mass below). A stricter interpretation does not equate the two, as the mass of a single molecule is not the same as the average of an ensemble. Because a mole of molecules may contain a variety of molecular masses due to natural isotopes, the average mass is usually not identical to the mass of any single molecule. The actual numerical difference can be very small when considering small molecules and the molecular mass of the most common isotopomer in which case the error only matters to physicists and a small subset of highly specialized chemists; however it is always more correct, accurate and consistent to use molar mass in any bulk stoichiometric calculations. The size of this error becomes much larger when considering larger molecules or less abundant isotopomers. The molecular mass of a molecule which happens to contain heavier isotopes than the average molecule in the sample can differ from the molar mass by several mass units. Also the molar mass comes into comparison with the number of particles contained within the structure by multiplying the number of moles by Avogadro's constant: NA=6.022 141 79(30)ˑ1023 mol−1.
Average mass
The average molecular mass (sometimes abbreviated as average mass) is another variation on the use of the term molecular mass. The average molecular mass is the abundance weighted mean (average) of the molecular masses in a sample. This is often closer to what is meant when "molecular mass" and "molar mass" are used synonymously and may have derived from shortening of this term. The average molecular mass and the molar mass of a particular substance in a particular sample are in fact numerically identical and may be interconverted by Avogadro's constant. It should be noted, however, that the molar mass is almost always a computed figure derived from the standard atomic weights, whereas the average molecular mass, in fields that need the term, is often a measured figure specific to a sample. Therefore, they often vary since one is theoretical and the other is experimental. Specific samples may vary significantly from the expected isotopic composition due to real deviations from earth's average isotopic abundances.
Computation
The molecular mass can be calculated as the sum of the individual isotopic masses (as found in a table of isotopes) of all the atoms in any molecule. This is possible because molecules are created by chemical reactions which, unlike nuclear reactions, have very small binding energies compared to the rest mass of the atoms (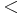 10−9) and therefore create a negligible mass defect. The use of average atomic masses derived from the standard atomic weights found on a standard periodic table will result in an average molecular mass, whereas the use of isotopic masses will result in a molecular mass consistent with the strict interpretation of the definition, i.e. that of a single molecule. However, any given molecule may contain any given combination of isotopes, so there may be multiple molecular masses for each chemical compound.
10−9) and therefore create a negligible mass defect. The use of average atomic masses derived from the standard atomic weights found on a standard periodic table will result in an average molecular mass, whereas the use of isotopic masses will result in a molecular mass consistent with the strict interpretation of the definition, i.e. that of a single molecule. However, any given molecule may contain any given combination of isotopes, so there may be multiple molecular masses for each chemical compound.
Measurement
The molecular mass can also be measured directly using mass spectrometry. In mass spectrometry, the molecular mass of a small molecule is usually reported as the monoisotopic mass, that is, the mass of the molecule containing only the most common isotope of each element. Note that this also differs subtly from the molecular mass in that the choice of isotopes is defined and thus is a single specific molecular mass of the many possible. The masses used to compute the monoisotopic molecular mass are found on a table of isotopic masses and are not found on a typical periodic table. The average molecular mass is often used for larger molecules since molecules with many atoms are unlikely to be composed exclusively of the most abundant isotope of each element. A theoretical average molecular mass can be calculated using the standard atomic weights found on a typical periodic table, since there is likely to be a statistical distribution of atoms representing the isotopes throughout the molecule. This however may differ from the true average molecular mass of the sample due to natural (or artificial) variations in the isotopic distributions.
The basis for determination of molecular weight according to the Staudinger method (since replaced by the more general Mark-Houwink equation[4]) is the fact that relative viscosity of suspensions depends on volumetric proportion of solid particles.
Unit type variation
The molar mass of a substance is the mass of 1 mol (the SI unit for the basis SI quantity amount of substance, having the symbol n) of the substance. This has a numerical value which is the average molecular mass of the molecules in the substance multiplied by Avogadro's constant approximately 6.022*1023. The most common units of molar mass are g/mol because in those units the numerical value equals the average molecular mass in units of u.
Conversion factor of average molecular mass to molar mass:
- molar mass = average molecular mass * ((1/6.022)*10-23g/u)*(6.022*1023/mol)
- or
- molar mass in g/mol= average molecular mass in u
(Note that these relations are true for theoretical and experimental values, but not between experimental and theoretical values. Molar mass is most often theoretical and average molecular mass is most often experimental)
The average atomic mass of natural hydrogen is 1.00794 u and that of natural oxygen is 15.9994 u; therefore, the molecular mass of natural water with formula H2O is (2 × 1.00794 u) + 15.9994 u = 18.01528 u. Therefore, one mole of water has a mass of 18.01528 grams. However, the exact mass of hydrogen-1 (the most common hydrogen isotope) is 1.00783, and the exact mass of oxygen-16 (the most common oxygen isotope) is 15.9949, so the mass of the most common molecule of water is 18.01056 u. The difference of 0.00472 u or 0.03% comes from the fact that natural water contain traces of water molecules containing, oxygen-17, oxygen-18 or hydrogen-2 (Deuterium) atoms. Although this difference is trivial in bulk chemistry calculations, it can result in complete failure in situations where the behavior of individual molecules matters, such as in mass spectrometry and particle physics (where the mixture of isotopes does not act as an average).
There are also situations where the isotopic distributions are not typical such as with heavy water used in some nuclear reactors which is artificially enriched with Deuterium. In these cases the computed values of molar mass and average molecular mass, which are ultimately derived from the standard atomic weights, will not be the same as the actual molar mass or average molecular mass of the sample. In this case the mass of deuterium is 2.0136 u and the average molecular mass of this water (assuming 100% deuterium enrichment) is (2 × 2.0136 u) + 15.9994 u = 20.0266 u. This is a very large difference of ~11% error from the expected average molecular mass based on the standard atomic weights. Furthermore the most abundant molecular mass is actually slightly less than the average molecular mass since oxygen-16 is still the most common. (2 × 2.0136 u) + 15.9949 u = 20.0221 u. Although this is an extreme artificial example, natural variation in isotopic distributions do occur and are measurable. For example, the atomic weight of lithium as found by isotopic analysis of 39 lithium reagents from several manufacturers varied from 6.939 to 6.996.[5]
See also
- Avogadro's number
- Atomic mass unit
- Absolute molar mass
- Monoisotopic mass
- gram-molecular weight
- Molar mass distribution
- Polymer