Malacostraca
| Malacostraca Fossil range: Cambrian–Recent |
|
|---|---|
 |
|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Crustacea |
| Class: | Malacostraca Latreille, 1802 |
| Subclasses | |
|
Eumalacostraca |
|
The Malacostraca (Greek: μαλακό όστρακο - "soft shell") are the largest class of crustaceans and include most of the animals that non-experts recognize as crustaceans, including decapods (such as crabs, lobsters and shrimp), stomatopods (mantis shrimp) and euphausiids (krill). They also include the amphipods and the only substantial group of land-based crustaceans, the isopods (woodlice and related species). With more than 22,000 members, this group represents two thirds of all crustacean species and contains all the larger forms. The first malacostracans appeared in the Cambrian [1].
The phylogeny of this group of organisms is debated [2]. Recent molecular studies (18S [3] and 28S [4]) have even disputed the monophyly of the Peracarida by removing the Mysida and have firmly disproven the monophyly of the Edriophthalma (Isopoda and Amphipoda) and the Mysidacea (Mysida, Lophogastrida and Pygocephalomorpha).
Contents |
Morphology
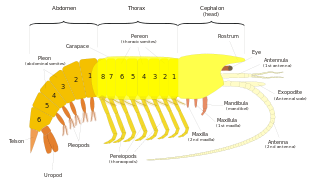
Their characteristics include:
- 3 tagmata: Head (5 segments), thorax (8 segments) and abdomen (6 segments (7 segments in Phyllocarida)).
- The head has 5 segments, with a pair of antennules and a pair of antennae, as well as 3 mouthparts, comprising the mandibles, the maxillula and the maxilla.
- There are 8 thoracic segments. The cephalothorax is covered by a carapace form via fusion of 3 of them, letting the 5 other uncovered.
- They usually have 8 pairs of thoracic legs (thoracopods), of which the first pair or several pairs are often modified into feeding appendages called maxillipeds. The first pair of legs behind the maxillipeds is often modified into pincers.
- The abdomen has 6 segments (7 segments in Phyllocarida). The appendages are called pleopods and are usually natatory. In Isopoda the pleopods are used for respiration.
- Fixation of gonopores on 6th segment for the female and on the 8th segment for the male.
- They have compound stalked or sessile eyes.
- They have a two-chambered stomach.
- They have a centralized nervous system.
Classification


Martin and Davis [5] present the following classification of living malacostracans into orders, to which extinct orders have been added, indicated by †.
Class Malacostraca Latreille, 1802
- Subclass Phyllocarida Packard, 1879
-
-
- †Order Archaeostraca Claus 1888
- †Order Hoplostraca Schram, 1973
- †Order Canadaspidida Novožilov in Orlov, 1960
- Order Leptostraca Claus, 1880
-
- Subclass Hoplocarida Calman, 1904
-
-
- †Order Aeschronectida Schram, 1969
- †Order Archaeostomatopoda Schram, 1969
- Order Stomatopoda Latreille, 1817 (mantis shrimp)
-
- Subclass Eumalacostraca Grobben, 1892
- Superorder Syncarida Packard, 1885
- †Order Palaeocaridacea Brooks, 1979
- Order Bathynellacea Chappuis, 1915
- Order Anaspidacea Calman, 1904
- Superorder Peracarida Calman, 1904
- Order Spelaeogriphacea Gordon, 1957
- Order Thermosbaenacea Monod, 1927
- Order Lophogastrida Sars, 1870
- Order Mysida Haworth, 1825 (opossum shrimp)
- Order Mictacea Bowman, Garner, Hessler, Iliffe & Sanders, 1985
- Order Amphipoda Latreille, 1816
- Order Isopoda Latreille, 1817 (including woodlice, slaters)
- Order Tanaidacea Dana, 1849
- Order Cumacea Krøyer, 1846 (hooded shrimp)
- Superorder Eucarida Calman, 1904
- Order Euphausiacea Dana, 1852 (krill)
- Order Amphionidacea Williamson, 1973
- Order Decapoda Latreille, 1802 (crabs, lobsters, shrimp and others)
- Superorder Syncarida Packard, 1885
Phylogeny
Although this class is united by a number of well defined and documented features as previously mention, which were recognised a century ago by Calman (1904)[6], the phylogenetic relationship (the evolutionary tree) of the orders which compose this class is unclear due to the vast diversity present in their morphology. Recently molecular studies have attempted to infer the phylogeny of this clade [7][8][9], resulting in phylogenies which have a limited amount of morphological support [10], to resolve a well-supported eumalacostracan phylogeny,it will be necessary to look beyond the most commonly utilized sources of data (nuclear ribosomal and mitochondrial sequences) to obtain a robust tree in the future.[11]
Features
The Malacostraca is assumed to be monophyletic due to several common morphological traits which are present throughout the group and due to molecular studies that have also confirmed it [12].
William T. Calman in 1904 and 1909 described these common morphological features and introduced the major taxonomic subdivisions of the Malacostraca which are still in use today: he divided the Malacostraca in two subclasses the Phyllocarida and the Eumalacostraca, which is further subdivided into four superorders: Eucarida, Peracarida, Hoplocarida and Syncarida.[13]
W.T. Calman coined the term caridoid facies for the common eumalacostracan (shrimp-like) features; the most important of these is the constant number of segments in each tagma: members of this class have five segments in the cephalon, eight thoracic segments (thoracomeres) and six segments in the pleon and possess a telson, which forms a characteristic tail fan when the uropods are present. Many other characteristic features are present but their presence varies amongst lineages; one notable ancestral feature which varies is the carapace, which may be absent, reduced or well developed covering the whole cephalothorax. Furthermore, Richter, S., & Scholtz, G. (2001)[14] list five separate unique eumalacostracan features which taken together form a strong argument in favour of the monophyly of the Eumalacostraca.
However debate arises in the relationship between the subdivisions of the Malacostraca, due to the presence of several contrasting features.

the traditional basal malacostracans

The Phyllocarida is a group of about 36 small marine species that are distributed across planet and possess a characteristic large bivalve carapace and an elongated abdomen with no uropods. This group is believed to be the most primitive malacostracan group, due to the fact that they lack some of the caridoid facies, such as the presence of seven abdominal segments (eight if telson is included). Furthermore one study by Wills, M.A. [15] places them as a sister branch to the Cephalocarida and basal to a Maxillopoda + Eumalacostraca clade and therefore making the Malacostraca paraphyletic.
The Eumalacostraca — the malacostraca minus the phyllocarida — were subdivided on the basis of several features, although which group is basal is unclear. Several authors, such as Siewin (1963), believe that the Syncarida is the most basal group due to the absence of morphological traits that are present in the remaining eumalacostracans; in addition, the Syncarida are distributed worldwide in reclusive habitats such as interstitial and groundwater, whereas their extensive fossil record shows that they were once marine, implying that the species present today are remnants of a more abundant group.
A second problematic group often attributed to be basal in the Eumalacostraca clade is the Hoplocarida. This group is composed of 200 species commonly called mantis shrimps, which are found in shallow tropical and subtropical marine habitats that have adapted to a predatorial life thanks to their specialized large second pair of thoracopods (thoracic appendices), raptorial legs, which are used to capture prey, in fact their name is a combination of Greek words meaning “armed shrimp”. Its precise location amongst the Malacostraca is unclear and has been proposed to be a sister group to the remaining eumalacostracans due to its ancient fossil record [14][16][17] but it has also been placed either sister to the Eucarida [15] or even inside the Eucarida by molecular studies[8]. In fact, the Malacostracan has a well-documented fossil record, that, although patchy or missing entirely (ghost lineage) for certain clades, offer a unique opportunity to analyse the morphology of the ancestral taxa of a clade or a dead-end sister taxa (plesion), whose age (determined by its stratigraphy) gives an estimate of how long has a group been around. However, the major limitation to fossilized samples is that typically the soft parts do not fossilize and are therefore lost, as a consequence a much more limited amount of information that can be gathered. Furthermore some taxa may not fossilize well, and therefore leave no trace even though they existed, when this occurs in the fossil record, the period were the taxa are expected to appear is called a ghost range.
Eucarida
Eucarida is a diverse and abundant group, whose members have a carapace which is fused to the thoracic segments to form a cephalothorax. The Eucarida is divided into three orders, the Euphausiacea, the Decapoda and the Amphionidacea.
The members of the Euphausiacea are commonly called krill and are all marine shrimp-like species whose pleopods (abdominal appendages) function as swimmerets, they swarm and mostly feed on plankton, this group is composed of only 90 species, but some of these are one of the most abundant species on the planet, in fact, it is estimated that the biomass of the Antarctic krill Euphausia superba' is 500 million tons [18].
The Decapoda is a group with 18,000 species which have 5 pairs of thoracopods and a well developed carapace that covers the gills (which are exposed in krill). Many of these species have common names and are often eaten. The decapods are further subdivided on the basis of the gill structure into two suborders Dendrobranchiata (prawns) and Pleocyemata, which is further subdivided into several infraorders, such as the Caridea (true shrimps), the Stenopodidea (boxer shrimp) and the Anomura and the Brachyura (Crabs) and so forth, although some authors [16][15] use alternative groupings for these three, Eukyphida, Euzygida and Reptantia (crabs and other decapods), respectively. In addition, there is an enigmatic eucarid species, Amphionides reynaudii, which is the sole representative of its order, but due to the loss of several features resulting from its small size, its classification has been unclear[13].
Peracarida
The other major malacostracan superorder, the Peracarida, is highly diverse in habit, size and shape and contains 21,500 species, but this number is a gross underestimate as the number of described species has tripled in the past 20 years[10]. Most authors studying morphological characters propose a monophyletic Peracarida which forms a well supported subtree that is sister to a Eucarida subtree, one paper is an exception [14] and proposes that the Peracarida is derived from a polyphyletic Eucarida.
With the exception of thermosbaenacean species, a characteristic of the members of this group is that they brood their young in a marsupium formed by branches (endites) of their thoracopods, called oostegites [13]. The Peracarida is divided into nine orders (Isopoda, Amphipoda, Mysida, Lophogastrida, Cumacea, Tanaidacea, Mictacea, Thermosbaenacea and Spelaeogriphacea), although some authors prefer to unite two pairs of orders with similar organisms, which are the Mysidacea, formed by the Mysida and the Lophogastrida, and the Edriophthalma, formed by the Isopoda and the Amphipoda [16].
The members of the Mysida and the Lophogastrida have several common features: they are shrimp-like with compound stalked eyes and have a carapace which covers most of the thorax but does not fuse with the last four thoracic segments (as instead is seen in the Eucarida), they possess well developed thoracopods (for swimming) and tail fan, they also have similar behaviour (swarming) and foregut structure [13]; Pygocephalomorph, a fossil from the Permian, appears similar to the Lophogastrida, which in addition to other factors, has traditionally allowed the Lophogastrida to be identified as the more primitive Mysidacea. However, the monophyly of the Mysidacea been recently disputed [13][8][9], due to molecular data and several differences, one profound difference between the two taxa is that in the Mysida the carapace acts as a respiratory surface due to the absence of gills, which are however present in the Lophogastrida. When they are considered together many authors have put Mysidacea basal to the remaining peracarids ((paraphyletic)Ruppert & Barnes, 1994; [15][16] ; (monophyletic)[17]), others have even either grouped them with Euphausiacea to form the Schizopoda[19] or made them basal to a eucarid subtree [20] (paraphyletic).
The Isopoda and the Amphipoda are two of the largest pericarid groups, they both lack a carapace, possess sessile compound eyes and lack a sharp demarcation between thoracic and abdominal segments, but however differ in several features, such as gills.
The Isopoda contains 10,000 species, the organisms are dorsoventrally flattened and occupy not only marine and freshwater habitats, but even terrestrial (woodlice) for which they developed a thickened cuticle and gas exchange organs, allowing them to live even in arid regions. The Amphipoda is a highly diverse group of 8,000 species, ranging from the Caprellida with a long and narrow body shape (skeleton shrimp) to the shrimp-like Gammaridea (scuds and sand hoppers).
The position of the Isopoda and the Amphipoda amongst the Peracarida is also debated, some authors support a derived united group (Edriophthalma) which is either monophyletic [16] [15] [17] or paraphyletic (Wheeler, 1998), others support a basal paraphyletic Isopoda and Amphipoda group (Watling, 1999); however, other authors believe that several features that unite the Isopoda and the Amphipoda are homoplasious and that the two groups reside with different groups: one[14] proposed a basal Amphipoda to a clade formed from Isopoda + Tanaidacea and Cumacea + Mictacea + Speleogriphacea , while some older phylogenetic trees [21][22] place the Amphipoda and the Mysidacea basal to the Peracarida (without the Thermosbaenacea) either as a polyphyletic or a monophyletic group, whereas the Isopoda are in a derived clade with Tanaidacea.
Recent molecular studies by Jarman et al. (2000)[7], Spears et al. (2005)[8] and Meland & Willassen (2007)[9] (which was derived from Spears et al., (2005) by adding 22 mysidacean taxa to those 26 taxa) suggest a phylogeny with some elements similar to that proposed by Richter & Scholtz (2001)[14], but disprove the monophyly of both the Edriophthalma and the Mysidacea and do not possess a basal eumalacostracan taxa or a basal peracarid taxa (the Hoplocarida and the Syncarida, respectively in [14]): The Amphipoda (with Spleogriphacea) form a clade with the Lophogastrida, the Isopoda are in a derived clade with the Cumacea and Tanaidacea, while most importantly the Mysida in all three analyses falls basal to the non-Peracarida subtree [8][9][7], which however has a limited morphological support (Poore, 2005).
The remaining Peracarida orders are the cryptic and either moderately abundant, Cumacea and Tanaidacea, or are extremely rare and relictual, Mictacea, Spelaeogriphacea, and Thermosbaenacea.
There are about 1,600 members of the Cumacea, these are small burrowing crustaceans which have a characteristic large bulbous carapace (covering three thoracic segments) and an elongated abdomen which finishes in a pleotelson with stylus-like uropods, in fact due to their peculiar shape they are sometimes called hooded shrimp. Tanaidacea is a group of 1,500 species which are small burrowing or tube-dwelling crustaceans with a short carapace (covering two thoracic segments) that possess a pair of chelate second thoracopods (gnathopods). Only three extant and two fossil speleogriphacean species have been found, these are blind cave-dwelling species with a short carapace (one thoracic segment); while the Mictacea is a group erected only two decades ago, and to date, five species have been found, Mictocaris halope (cave dwelling) and four species in the Hursutiidae family, in the genera Hirsutia (at 1,000 meter depths) and Thetispelecaris (submarine caves), these blind species lack a carapace but have a well developed headshield, and have reduced pleopods. This enigmatic group is believed by some authors not to be monophyletic, in fact one author proposed that Mictocaris halope should be grouped with the Spelaeogriphacea, forming the Cosinzeneacea.[23]
The Thermosbaenacea is a group of 11 species found in hot springs, caves and groundwater that has the peculiarity that its brooding pouch is formed by its extended carapace and not by modified thoracopod endites as occurs in the remaining peracarids, for this reason some authors have removed this group from the Peracarida and placed it in its own superorder, the Pancarida, basal to the Pericarida [21][22][14].
Molecular studies
The major feature that emerges from the picture of the phylogeny of the Malacostraca is that their diversity has resulted in several studies proposing dramatically different phylogenetic trees. With the advent of DNA sequencing, molecular studies have not helped a particular evolutionary model based on morphology to become the accepted one, but rather they have added more uncertainty to the accepted phylogenetic relationships: in fact not only they contradicted several morphological studies, but they also questioned the monophyly of the Eucarida and the Peracarida, in particular regarding the positions of the Mysida the Syncarida and the Hoplocarida, furthermore the molecular studies to date are only three, two of which overlap [8][9] and concentrate on the Peracarida, while the third [7] uses a limited amount of taxa.
References
- ↑ Frederick R. Schram (1974). "Convergences Between Late Paleozoic and Modern Caridoid Malacostraca". Systematic Zoology (Society of Systematic Biologists) 23 (3): 323–332. doi:10.2307/2412539. http://www.jstor.org/stable/2412539.
- ↑ Frederick R. Schram (1986). Crustacea. Oxford University Press. ISBN 0-19-503742-1.
- ↑ K. Meland & E. Willassen (2007). "The disunity of "Mysidacea" (Crustacea)". Molecular Phylogenetics and Evolution 44 (3): 1083–1104. doi:10.1016/j.ympev.2007.02.009. PMID 17398121. http://linkinghub.elsevier.com/retrieve/pii/S1055790307000310.
- ↑ S. N. Jarman, S. Nicol, N. G. Elliott & A. McMinn (2000). "28S rDNA evolution in the Eumalacostraca and the phylogenetic position of krill". Molecular Phylogenetics and Evolution 17 (1): 26–36. doi:10.1006/mpev.2000.0823. PMID 11020302. http://linkinghub.elsevier.com/retrieve/pii/S1055790300908236.
- ↑ Joel W. Martin & George E. Davis (2001) (PDF). An Updated Classification of the Recent Crustacea. Natural History Museum of Los Angeles County. pp. 132 pp. http://atiniui.nhm.org/pdfs/3839/3839.pdf.
- ↑ Calman, W. (1904). On the classification of the Crustacea Malacostraca. The Annals of Magazine of Natural History , (7)13, 144-158.
- ↑ 7.0 7.1 7.2 7.3 7.4 Jarman, S. N., Nicol, S., Elliott, N. G., & McMinn, A. (2000). 28S rDNA Evolution in the Eumalacostraca and the Phylogenetic Position of Krill. Molecular Phylogenetics and Evolution , 17(1), 26–36.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Spears, T., DeBry, R., Abele, L., & Chodyla, K. (2005). Peracarid monophyly and interordinal phylogeny inferred from nuclear small-subunit ribosomal DNA sequences (Crustacea: Malacostraca: Peracarida). Proceedings of the biological society of Washinghton , 118(1) , 117-157.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Meland, K., & Willassen, E. (2007). The disunity of “Mysidacea” (Crustacea). Molecular Phylogenetics and Evolution , 44, 1083–1104.
- ↑ 10.0 10.1 Poore, G. C. (2005). Peracarida: monophyly, relationships and evolutionary. Nauplius , 13(1), 1-27.
- ↑ Jenner RA, Ní Dhubhghaill C, Ferla MP, Wills MA. Eumalacostracan phylogeny and total evidence: limitations of the usual suspects.BMC Evol Biol. 2009 Jan 27;9:21.
- ↑ Hassanin, A. (2006). Phylogeny of Arthropoda inferred from mitochondrial sequences: Strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution. Molecular Phylogenetics and Evolution , 38, 100–116.
- ↑ 13.0 13.1 13.2 13.3 13.4 Brusca, R., & Brusca, G. (2003). Invertebrates (2nd Edition ed.). Sunderland, Mass. : Sinauer Associates.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Richter, S., & Scholtz, G. (2001). Phylogenetic analysis of the Malacostraca (Crustacea). J. Zool. Syst. Evol. Research , 39, 113-136.
- ↑ 15.0 15.1 15.2 15.3 15.4 Wills, M. A. (1998). A phylogeny of recent and fossil Crustacea derived from morphological characters. In R. F. Thomas, Arthropod Relatioships (Vol. 55). Chapman and Hall, London.
- ↑ 16.0 16.1 16.2 16.3 16.4 Schram, F. R. (1986). Crustacea. Oxford University Press.
- ↑ 17.0 17.1 17.2 Watling, L., Hof, C. H., & Schram, F. R. (2000). The Place of the Hoplocarida in the Malacostracan Pantheon. Journal of Crustacean Biology , (20)2, 1-11.
- ↑ Martin, J., & Davis, G. (2001). An updated classification of the recent Crustacea. (Vols. Natural History Museum of Los Angeles County, Science Series 39).
- ↑ Sars, G. O. (1870). Carcinologiske Bidrag til Norges Fauna over de ved Norges Kysters forekommende Mysider. (Vol. 1). Christiana: Brøgger & Christies Bogtrykkeri.
- ↑ Watling, L. (1999). Towards understanding the relationship of the peracaridan orders: the necessity of determining exact homologies. Crustaceans and the Biodiversity Crisis. Procedings of the fourth International Crustacean congress, Amsterdam, (pp. 73-89).
- ↑ 21.0 21.1 Siewing, R. (1963). Studies in malacostracan morphology: results and problems. In H. B. Whittington, & W. D. Rolfe, Phylogeny and Evolution of Crustacea. (pp. 85-103). Cambridge, Mass: Museum of Comparative Zoology.
- ↑ 22.0 22.1 Pires, A. M. (1987). Potiicoara brasiliensis: a new genus and species of Spelaeogriphacea (Crustacea: Peracarida) from Brasil with a phylogenetic analysis of the Peracarida. Journal of Natural History , 21, 225-238.
- ↑ Gutu, M. 1998. Description of three new species of Tanaidacea (Crustacea) from the Tanzanian coasts.-- Travaux du Muséum National d´Histoire naturelle "Grigore Antipa" 40: 179-209
External links
- Malacostraca image key - Guide to the marine zooplankton of south eastern Australia, Tasmanian Aquaculture and Fisheries Institute
|
|||||||||||||||||