Lymphocyte

A lymphocyte is a type of white blood cell in the vertebrate immune system.[1]
Under the microscope, lymphocytes can be divided into large granular lymphocytes and small lymphocytes. Large granular lymphocytes include natural killer cells (NK cells). Small lymphocytes consist of T cells and B cells.
Contents |
Types

The three major types of lymphocyte are T cells, B cells and natural killer (NK) cells.
Natural killer cells
NK cells are a part of innate immune system and play a major role in defending the host from both tumors and virally infected cells. NK cells distinguish infected cells and tumors from normal and uninfected cells by recognizing level changes of a surface molecule called MHC (major histocompatibility complex) class I. NK cells are activated in response to a family of cytokines called interferons. Activated NK cells release cytotoxic (cell-killing) granules which then destroy the altered cells.[2] They were named "natural killer cells" because of the initial notion that they do not require prior activation in order to kill cells which are missing MHC class I.
T cells and B cells
T cells (Thymus cells) and B cells (bone cells) are the major cellular components of the adaptive immune response. T cells are involved in cell-mediated immunity whereas B cells are primarily responsible for humoral immunity (relating to antibodies). The function of T cells and B cells is to recognize specific “non-self” antigens, during a process known as antigen presentation. Once they have identified an invader, the cells generate specific responses that are tailored to maximally eliminate specific pathogens or pathogen infected cells. B cells respond to pathogens by producing large quantities of antibodies which then neutralize foreign objects like bacteria and viruses. In response to pathogens some T cells, called T helper cells, produce cytokines that direct the immune response while other T cells, called cytotoxic T cells, produce toxic granules that induce the death of pathogen infected cells. Following activation, B cells and T cells leave a lasting legacy of the antigens they have encountered, in the form of memory cells. Throughout the lifetime of an animal these memory cells will “remember” each specific pathogen encountered, and are able to mount a strong response if the pathogen is detected again.
Development
_diagram.png)
Mammalian stem cells differentiate into several kinds of blood cell within the bone marrow.[3] This process is called haematopoiesis. All lymphocytes originate, during this process, from a common lymphoid progenitor before differentiating into their distinct lymphocyte types. The differentiation of lymphocytes follows various pathways in a hierarchical fashion as well as in a more plastic fashion. The formation of lymphocytes is known as lymphopoiesis. B cells mature into B lymphocytes in the bone marrow[4], while T cells migrate to and mature in a distinct organ, called the thymus. Following maturation, the lymphocytes enter the circulation and peripheral lymphoid organs (e.g. the spleen and lymph nodes) where they survey for invading pathogens and/or tumor cells.
The lymphocytes involved in adaptive immunity (i.e. B and T cells) differentiate further after exposure to an antigen; they form effector and memory lymphocytes. Effector lymphocytes function to eliminate the antigen, either by releasing antibodies (in the case of B cells), cytotoxic granules (cytotoxic T cells) or by signaling to other cells of the immune system (helper T cells). Memory cells remain in the peripheral tissues and circulation for an extended time ready to respond to the same antigen upon future exposure.
They live weeks to several years[5] to a whole lifetime, which is very long compared to other leukocytes.
Characteristics
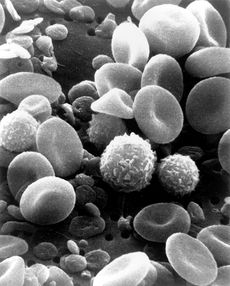
Microscopically, in a Wright's stained peripheral blood smear, a normal lymphocyte has a large, dark-staining nucleus with little to no eosinophilic cytoplasm. In normal situations, the coarse, dense nucleus of a lymphocyte is approximately the size of a red blood cell (about 7 micrometres in diameter).[3] Some lymphocytes show a clear perinuclear zone (or halo) around the nucleus or could exhibit a small clear zone to one side of the nucleus. Polyribosomes are a prominent feature in the lymphocytes and can be viewed with an electron microscope.[3] The ribosomes are involved in protein synthesis allowing the generation of large quantities of cytokines and immunoglobulins by these cells.
It is impossible to distinguish between T cells and B cells in a peripheral blood smear.[3] Normally, flow cytometry testing is used for specific lymphocyte population counts. This can be used to specifically determine the percentage of lymphocytes that contain a particular combination of specific cell surface proteins, such as immunoglobulins or cluster of differentiation (CD) markers or that produce particular proteins (for example, cytokines using intracellular cytokine staining (ICCS)). In order to study the function of a lymphocyte by virtue of the proteins it generates, other scientific techniques like the ELISPOT or secretion assay techniques can be used.[2]
| CLASS | FUNCTION | PROPORTION | PHENOTYPIC MARKER(S) |
| NK cells | Lysis of virally infected cells and tumour cells | 7% (2-13%) | CD16 CD56 but not CD3 |
| Helper T cells | Release cytokines and growth factors that regulate other immune cells | 46% (28-59%) | TCRαβ, CD3 and CD4 |
| Cytotoxic T cells | Lysis of virally infected cells, tumour cells and allografts | 19% (13-32%) | TCRαβ, CD3 and CD8 |
| γδ T cells | Immunoregulation and cytotoxicity | TCRγδ and CD3 | |
| B cells | Secretion of antibodies | 23% (18-47%) | MHC class II, CD19 and CD21 |
In the circulatory system they move from lymph node to lymph node. This contrasts with macrophages, which are rather stationary in the nodes.
Lymphocytes and disease
A lymphocyte count is usually part of a peripheral complete blood cell count and is expressed as percentage of lymphocytes to total white blood cells counted.
A general increase in the number of lymphocytes is known as lymphocytosis whereas a decrease is lymphocytopenia.
High
An increase in lymphocyte concentration is usually a sign of a viral infection (in some rare case, leukemias are found through an abnormally raised lymphocyte count in an otherwise normal person).
Low
A low normal to low absolute lymphocyte concentration is associated with increased rates of infection after surgery or trauma.
One basis for low T cell lymphocytes occurs when the human immunodeficiency virus (HIV) infects and destroys T cells (specifically, the CD4+ subgroup of T lymphocytes). Without the key defense that these T cells provide, the body becomes susceptible to opportunistic infections that otherwise would not affect healthy people. The extent of HIV progression is typically determined by measuring the percentage of CD4+ T cells in the patient's blood. The effects of other viruses or lymphocyte disorders can also often be estimated by counting the numbers of lymphocytes present in the blood.
Blood content

See also
- Addressin
- Anergy
- Complete blood count
- Cytotoxicity
- Human leukocyte antigen
- Lymphoproliferative disorders
- Reactive lymphocyte
- Secretion assay
References
- ↑ "Dorlands Medical Dictionary:lymphocyte". http://www.mercksource.com/pp/us/cns/cns_hl_dorlands_split.jspzQzpgzEzzSzppdocszSzuszSzcommonzSzdorlandszSzdorlandzSzfivezSz000061991zPzhtm. Retrieved 2009-01-27.
- ↑ 2.0 2.1 Janeway, Charles; Paul Travers, Mark Walport, and Mark Shlomchik (2001). Immunobiology; Fifth Edition. New York and London: Garland Science. ISBN 0-8153-4101-6. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=10..
- ↑ 3.0 3.1 3.2 3.3 Abbas AK and Lichtman AH (2003). Cellular and Molecular Immunology (5th ed.). Saunders, Philadelphia. ISBN 0-7216-0008-5.
- ↑ Kumar, Abbas, Fausto. Pathologic Basis of Disease (7th ed.).
- ↑ Semester 4 medical lectures at Uppsala University 2008 by Leif Jansson
- ↑ Berrington, J. E.; Barge, D; Fenton, AC; Cant, AJ; Spickett, GP (2005-05). "Lymphocyte subsets in term and significantly preterm UK infants in the first year of life analysed by single platform flow cytometry". Clin Exp Immunol 140 (2): 289–292. doi:10.1111/j.1365-2249.2005.02767.x. PMID 15807853. PMC 1809375. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1809375&rendertype=table&id=tbl3.
External links
|
||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||