Lipoprotein
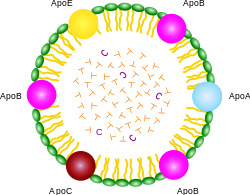
ApoA, ApoB, ApoC, ApoE (apolipoproteins); T (triacylglycerol); C (cholesterol); green (phospholipids)
A lipoprotein is a biochemical assembly that contains both proteins and lipids whose function is to transport water-insoluble lipids in the water-based bloodstream. The lipids or their derivatives may be covalently or non-covalently bound to the proteins. Many enzymes, transporters, structural proteins, antigens, adhesins and toxins are lipoproteins. Examples include the high density (HDL) and low density (LDL) lipoproteins which enable fats to be carried in the blood stream, the transmembrane proteins of the mitochondrion and the chloroplast, and bacterial lipoproteins.[1]
Contents |
Function
The function of lipoprotein particles is to transport water-insoluble lipids (fats) and cholesterol around the body in the blood.
All cells use and rely on fats and, for all animal cells, cholesterol as building blocks to create the multiple membranes which cells use to both control internal water content, internal water soluble elements and to organize their internal structure and protein enzymatic systems.
The lipoprotein particles have hydrophilic groups of phospholipids, cholesterol and apoproteins directed outward. Such characteristics makes them soluble in the salt water-based blood pool. Triglyceride-fats and cholesterol esters are carried internally, shielded from the water by the phospholipid monolayer and the apoproteins.
The interaction of the proteins forming the surface of the particles with (a) enzymes in the blood, (b) with each other, and (c) with specific proteins on the surfaces of cells determine whether triglycerides and cholesterol will be added to or removed from the lipoprotein transport particles.
Regarding atheroma development and progression as opposed to regression, the key issue has always been cholesterol transport patterns, not cholesterol concentration itself.
Transmembrane lipoproteins
The lipids are often an essential part of the complex, even if they seem to have no catalytic activity by themselves. To isolate transmembrane lipoproteins from their associated membranes, detergents are often needed.
Classification
By density
Lipoproteins may be classified as follows, listed from larger and less dense ones to smaller and denser ones. Lipoproteins are larger and less dense, if they consist of more fat than of protein. They are classified on the basis of electrophoresis and ultracentrifugation.
- Chylomicrons carry triglycerides (fat) from the intestines to the liver, skeletal muscle, and to adipose tissue.
- Very low density lipoproteins (VLDL) carry (newly synthesised) triacylglycerol from the liver to adipose tissue.
- Intermediate density lipoproteins (IDL) are intermediate between VLDL and LDL. They are not usually detectable in the blood.
- Low density lipoproteins (LDL) carry cholesterol from the liver to cells of the body. LDLs are sometimes referred to as the "bad cholesterol" lipoprotein.
- High density lipoproteins (HDL) collect cholesterol from the body's tissues, and bring it back to the liver. HDLs are sometimes referred to as the "good cholesterol" lipoprotein.
| Density (g/mL) | Class | Diameter (nm) | % protein | % cholesterol | % phospholipid | % triacylglycerol |
| >1.063 | HDL | 5–15 | 33 | 30 | 29 | 4 |
| 1.019–1.063 | LDL | 18–28 | 25 | 50 | 21 | 8 |
| 1.006–1.019 | IDL | 25–50 | 18 | 29 | 22 | 31 |
| 0.95–1.006 | VLDL | 30–80 | 10 | 22 | 18 | 50 |
| <0.95 | Chylomicrons | 100-1000 | <2 | 8 | 7 | 84 |
Alpha and beta
It is also possible to classify lipoproteins as "alpha" and "beta", according to the classification of proteins in serum protein electrophoresis. This terminology is sometimes used in describing lipid disorders such as Abetalipoproteinemia.
Lipoprotein(a)
Lipoprotein(a) – Lp(a), Cardiology diagnostic tests
- < 14 mg/dL : Normal
- 14-19 mg/dL : ?
- > 19 mg/dL : High risk
How to lower: aerobic exercise, niacin, aspirin, guggulipid.[3]
Metabolism
The handling of lipoproteins in the body is referred to as "lipoprotein metabolism'. It is divided into two pathways, exogenous and endogenous, depending in large part on whether the lipoproteins in question are composed chiefly of dietary (exogenous) lipids or whether they originated in the liver (endogenous).
Exogenous pathway
Epithelial cells lining the small intestine readily absorb lipids from nutritive substances. These lipids, including triglycerides, phospholipids, and cholesterol, are assembled with apolipoprotein B-48 into chylomicrons. These nascent chylomicrons are secreted from the intestinal epithelial cells into the lymphatic circulation in a process that depends heavily on apolipoprotein B-48. As they circulate through the lymphatic vessels, nascent chylomicrons bypass the liver circulation and are drained via the thoracic duct into the bloodstream.
In the bloodstream, HDL particles donate apolipoprotein C-II and apolipoprotein E to the nascent chylomicron; the chylomicron is now considered mature. Via apolipoprotein C-II, mature chylomicrons activate lipoprotein lipase (LPL), an enzyme on endothelial cells lining the blood vessels. LPL catalyzes the hydrolysis of triacylglycerol (i.e. glycerol covalently joined to three fatty acids) that ultimately releases glycerol and fatty acids from the chylomicrons. Glycerol and fatty acids can then be absorbed in peripheral tissues, especially adipose and muscle, for energy and storage.
The hydrolyzed chylomicrons are now considered chylomicron remnants. The chylomicron remnants continue circulating until they interact via apolipoprotein E with chylomicron remnant receptors, found chiefly in the liver. This interaction causes the endocytosis of the chylomicron remnants, which are subsequently hydrolyzed within lysosomes. Lysosomal hydrolysis releases glycerol and fatty acids into the cell, which can be used for energy or stored for later use.
Endogenous pathway
The liver is another important source of lipoproteins, principally VLDL. Triacylglycerol and cholesterol are assembled with apolipoprotein B-100 to form VLDL particles. Nascent VLDL particles are released into the bloodstream via a process that depends upon apolipoprotein B-100.
As in chylomicron metabolism, the apolipoprotein C-II and apolipoprotein E of VLDL particles are acquired from HDL particles. Once loaded with apolipoproteins C-II and E, the nascent VLDL particle is considered mature.
Again like chylomicrons, VLDL particles circulate and encounter LPL expressed on endothelial cells. Apolipoprotein C-II activates LPL, causing hydrolysis of the VLDL particle and the release of glycerol and fatty acids. These products can be absorbed from the blood by peripheral tissues, principally adipose and muscle. The hydrolyzed VLDL particles are now called VLDL remnants or intermediate density lipoproteins (IDLs). VLDL remnants can circulate and, via an interaction between apolipoprotein E and the remnant receptor, be absorbed by the liver, or they can be further hydrolyzed by hepatic lipase.
Hydrolysis by hepatic lipase releases glycerol and fatty acids, leaving behind IDL remnants, called low density lipoproteins (LDL), which contain a relatively high cholesterol content. LDL circulates and is absorbed by the liver and peripheral cells. Binding of LDL to its target tissue occurs through an interaction between the LDL receptor and apolipoprotein B-100 or E on the LDL particle. Absorption occurs through endocytosis, and the internalized LDL particles are hydrolyzed within lysosomes, releasing lipids, chiefly cholesterol.
See also
- Apolipoprotein
- Lipid anchor
- Lipid anchored protein
- Vertical Auto Profile
References
- ↑ mrc-lmb.cam.ac.uk
- ↑ Biochemistry 2nd Ed. 1995 Garrett & Grisham
- ↑ Beyond Cholesterol, Julius Torelli MD, 2005 ISBN 0-312-34863-0 p.91
External links
- Lipoprotein Information, Diagrams, & Exercise
- Database of bacterial lipoproteins at mrc-lmb.cam.ac.uk
- Overview and diagram at washington.edu
- Lipoprotein assembly at wisc.edu
- Lipoprotein circulation at purdue.edu
- MeSH Lipoproteins
- MeSH Proteolipids
|
|||||||||||
|
||||||||||||||||||||||||||