Limonene
| Limonene | |
|---|---|
 |
 |
|
1-methyl-4-(1-methylethenyl)-cyclohexene
|
|
|
Other names
4-isopropenyl-1-methylcyclohexene
Racemic: DL-limonene; dipentene |
|
| Identifiers | |
| CAS number | 5989-27-5 |
|
SMILES
CC(=C)[C@@H]1CCC(=CC1)C
|
|
| Properties | |
| Molecular formula | C10H16 |
| Molar mass | 136.24 g/mol |
| Density | 0.8411 g/cm³ |
| Melting point |
-74.35 °C, 199 K, -102 °F |
| Boiling point |
176 °C, 449 K, 349 °F |
| Hazards | |
| R-phrases | R10 R38 R43 R50/53 |
| S-phrases | (S2) S24 S37 S60 S61 |
| NFPA 704 |
 2
1
0
|
| Flash point | 50 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Limonene is a colourless liquid hydrocarbon classified as a cyclic terpene possessing a strong smell of oranges. It is used in chemical synthesis as a precursor to carvone and as a renewably-based solvent in cleaning products.
Limonene takes its name from the lemon, as the rind of the lemon, like other citrus fruits, contains considerable amounts of this compound, which contributes to their smell. Limonene is a chiral molecule, and biological sources produce one enantiomer: the principal industrial source, citrus fruit, contains D-limonene ((+)-limonene), which is the (R)-enantiomer (CAS number 5989-27-5, EINECS number 227-813-5). Racemic limonene is known as dipentene.[1] D-Limonene is obtained commercially by extraction from orange peel with liquid CO2.
Contents |
Chemistry
Limonene is a relatively stable terpene and can be distilled without decomposition, although at elevated temperatures it cracks to form isoprene.[2] It oxidizes easily in moist air to produce carveol and carvone.[3] With sulfur, it undergoes dehydrogenation to p-cymene.
Reactions
Limonene occurs naturally as the (R)-enantiomer, but racemizes to dipentene at 300 °C. When warmed with mineral acid, limonene isomerizes to the conjugated diene α-terpinene (which can also easily be converted to p-cymene). Evidence for this isomerization includes the formation of Diels-Alder adducts between α-terpinene adducts and maleic anhydride.
It is possible to effect reaction at one of the double bonds selectively. Anhydrous hydrogen chloride reacts preferentially at the disubstituted alkene, whereas epoxidation with MCPBA occurs at the trisubstituted alkene.
In another synthetic method Markovnikov addition of trifluoroacetic acid followed by hydrolysis of the acetate gives terpineol.
The most widely practiced conversion of limonene is to carvone. The three step reaction begins with the regioselective addition of nitrosyl chloride across the trisubstituted double bond. This species is then converted to the oxime with base, and the hydroxylamine is removed to give the ketone-containing carvone.[4]
Biosynthesis
Limonene is formed from geranyl pyrophosphate, via cyclization of a neryl carbocation or its equivalent as shown.[5] The final step involves loss of a proton from the cation to form the alkene.
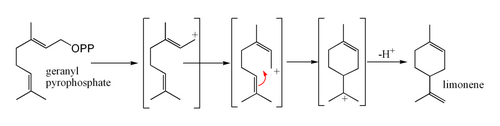
Uses
The major use of D-limonene is as a precursor to carvone.[4]
Limonene is common in cosmetic products. As the main odor constituent of citrus (plant family Rutaceae), D-limonene is used in food manufacturing and some medicines, e.g., bitter alkaloids, as a flavoring; it is also used as botanical insecticide.[6] It is added to cleaning products such as hand cleansers to give a lemon-orange fragrance (see orange oil). In contrast, L-limonene has a piney, turpentine-like odor.
Limonene is increasingly being used as a solvent for cleaning purposes, such as the removal of oil from machine parts, as it is produced from a renewable source (citrus oil, as a byproduct of orange juice manufacture). It also serves as a paint stripper when applied to painted wood. Limonene is also used as a solvent in some model airplane glues.
As it is combustible, limonene has also been considered as a biofuel.[7]
Safety
Limonene and its oxidation products are skin and respiratory irritants, and limonene-1,2-oxide (formed by aerial oxidation) is a known skin sensitizer. Most reported cases of irritation have involved long-term industrial exposure to the pure compound, e.g. during degreasing or the preparation of paints. However a study of patients presenting dermatitis showed that 3% were sensitized to limonene.[8]
Although once thought to cause renal cancer in rats, limonene now is considered by some researchers to be a significant chemopreventive agent [9] with potential value as a dietary anti-cancer tool in humans.[10] There is no evidence for carcinogenicity or genotoxicity in humans. The IARC classifies D-limonene under Class 3: not classifiable as to its carcinogenicity to humans.[8] The Carcinogenic Potency Project, however, estimates that it causes human cancer on a level roughly equivalent to that caused by exposure to caffeic acid via dietary coffee intake.[11]
No information is available on the health effects of inhalation exposure to D-limonene in humans, and no long-term inhalation studies have been conducted in laboratory animals.
D-Limonene is biodegradable, but due to its low flash point, it must be treated as hazardous waste for disposal.
Compendial status
- British Pharmacopoeia [12]
See also
- Citral
- Lemon
- Perfume allergy
Notes
- ↑ J. L. Simonsen (1947). The Terpenes. 1 (2nd ed.). Cambridge University Press.
- ↑ H. Pakdela, D. Panteaa and C. Roy (2001). "Production of dl-limonene by vacuum pyrolysis of used tires". J Anal Appl Pyrolysis 57 (1): 91–107. doi:10.1016/S0165-2370(00)00136-4.
- ↑ Source: European Chemicals Bureau.
- ↑ 4.0 4.1 Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg “Flavors and Fragrances“ in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_141.
- ↑ Mann, J. C.; Hobbs, J. B.; Banthorpe, D. V.; Harborne, J. B. (1994). Natural products: their chemistry and biological significance. Harlow, Essex, England: Longman Scientific & Technical. pp. 308–9. ISBN 0-582-06009-5.
- ↑ EPA R.E.D. Fact Sheet on Limonene, September 1994
- ↑ Cyclone Power to Showcase External Combustion Engine at SAE Event, Green Car Congress, 20 September 2007
- ↑ 8.0 8.1 "IARC Monographs on the evaluation of carcinogenic risks to humans". 1999. pp. 307–27. http://monographs.iarc.fr/ENG/Monographs/vol73/mono73-16.pdf.
- ↑ Crowell PL (March 1999). "Prevention and therapy of cancer by dietary monoterpenes". J. Nutr. 129 (3): 775S–8S. PMID 10082788. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=10082788.
- ↑ Tsuda H, Ohshima Y, Nomoto H, et al. (August 2004). "Cancer prevention by natural compounds". Drug Metab. Pharmacokinet. 19 (4): 245–63. doi:10.2133/dmpk.19.245. PMID 15499193. http://www.jstage.jst.go.jp/article/dmpk/19/4/19_245/_article.
- ↑ "Ranking Possible Cancer Hazards on the HERP Index". http://potency.lbl.gov/pdfs/herp.pdf. Retrieved 2007-03-19.
- ↑ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf. Retrieved 31 March 2010.
References
- E. E. Turner, M. M. Harris (1952). Organic Chemistry. London: Longmans, Green & Co..
- Wallach (1888). Annalen der Chemie 246: 221.
- Blumann & Zeitschel (1914). Berichte 47: 2623.
- Source: CSST Workplace Hazardous Materials Information System.
- Matura M, Goossens A, Bordalo O, et al. (November 2002). "Oxidized citrus oil (R-limonene): a frequent skin sensitizer in Europe". J. Am. Acad. Dermatol. 47 (5): 709–14. doi:10.1067/mjd.2002.124817. PMID 12399762. http://linkinghub.elsevier.com/retrieve/pii/S0190962202001500.