Lepidoptera
| Moths, butterflies and allies Fossil range: 199–0 Ma Jurassic – Recent |
|
|---|---|
 |
|
| A Giant Leopard Moth (Hypercompe scribonia) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Subclass: | Pterygota |
| Infraclass: | Neoptera |
| Superorder: | Endopterygota |
| Order: | Lepidoptera Linnaeus, 1758 |
| Suborders | |
|
Aglossata |
|
Lepidoptera (pronounced /ˌlɛpɪˈdɒptərə/) is a large order of insects that includes moths and butterflies (called lepidopterans). It is one of the most speciose orders in the world, encompassing moths and the three superfamilies of butterflies, skipper butterflies, and moth-butterflies and found virtually everywhere. Lepidoptera contains more than 180,000 species[1] in 128 families and 47 superfamilies. The name is derived from Ancient Greek λεπίδος (scale) and πτερόν (wing). Estimates of species suggest that the order may have more species and is among the four largest, successful orders, along with the Hymenoptera, Diptera, and the Coleoptera.[2]
Species of the order Lepidoptera are commonly characterized as being covered in scales, having two large compound eyes, and an elongated mouthpart called a proboscis. Almost all species have membranous wings, except for a few who have crossvein wings. The larvae are called caterpillars and are completely different in form, having a cylindrical body with a well developed head, mandible mouthparts, and from 0–11 (usually 8) legs.
The Lepidoptera have, over millions of years, evolved a wide range of wing patterns and coloration ranging from drab moths akin to the related order Trichoptera to the brightly colored and complex-patterned butterflies.[3] Accordingly, this is the most recognized and popular of insect orders with many people involved in the observing, study, collecting, rearing and commerce of these insects. A person who collects or studies this order is referred to as a lepidopterist. Many species of the order are of economic interest by virtue of the silk they produce, and serve an important natural role through pollination.
Contents |
Etymology
The word Lepidoptera comes from the Latin word for "scaly wing", from the Ancient Greek λεπίδος (Lepidos) meaning scale and πτερόν (pteron) meaning wing. Sometimes the term Rhopalocera is used to group the species that are butterflies, from the Ancient Greek ῥόπαλον (Rhopalon) and κέρας (kæras) meaning club and horn respectively; coming from the shape of the antennae of butterflies.
The origins of the common names of many species vary. The English word butterfly is from Old English buttorfleoge, with many variations in spelling. Other than that, the origin is unknown, however it could be derived from the pale yellow color of many species' wings (e.g., Yellow Sulfur: Pieridae) suggests the color of butter.[4][5](butterfly) The species of Heterocera are commonly called moths. The origins of the English word moth are more clear, which comes from Old English "moððe" (cf. Northumbrian "mohðe") from Common Germanic (compare Old Norse "motti", Dutch "Mot" and German "Motte" all meaning "moth"). Perhaps its origins are related to Old English "maða" meaning "maggot" or from the root of "midge" which until the 16th century was used mostly to indicate the larva, usually in reference to devouring clothes.[5](moth)
Distribution and diversity
Out of the more than 180,000 species described to date can be found virtually everywhere. Some 11,300 species are from North America, and 10,000 from Australia. Lepidoptera are found in a large variety of habitats, but almost always associated with higher plants, especially angiosperms (flowering plants).[6]
Morphology and physiology
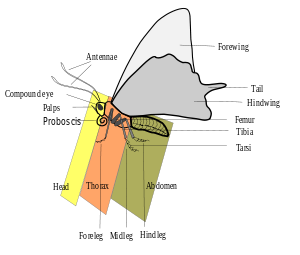
As in all insects, all species of Lepidoptera have an exoskeleton and segmented into three parts (head, thorax, abdomen) with scales and three pairs of legs. They also have two large compound eyes and an elongated mouthpart called a proboscis. Almost all species have membranous wings, except for a few who have crossvein wings. The larva are completely different in form, having a soft body with a well developed head, mandible mouthparts, and up to 11 pairs of legs (usually 8).[6]
Morphology
Head
The adult head is segmented into six segments, though controversial, and covered with hair-like scales. Lepidoptera have a long proboscis curled under their head, and have two large compound eyes which cover much of their head.[7] These compound eyes are made of a large number of hexagonal facets, or lenses. Each facet is connected to a lens-like cylinder that is attached to nerves leading to the brain.[8] Each facet subtends and forms the part of an ommatidium, or cluster of photoreceptor cells.[7]
Lepidoptera have long, segmented antennae that act as olfactory organs.[8] Lepidoptera antennae also have a Johnston's organ, a collection of sensory cells found in the pedicle (or second segment) of the antennae which perceives stretching between the pedicel and the rest of the antenna.[7] In the case of Monarch butterflies, it has been shown that antennae are necessary for proper time-compensated Sun compass orientation during migration, that antennal clocks exist in monarchs, and that they likely provide the primary timing mechanism for sun compass orientation.[9][10] In moths, males frequently have more feathery antennae than females, for detecting the female pheromones at a distance.
Caterpillars have very different heads than adult butterflies and moths, with a hard and well developed head cap for protection and mandible mouthparts designed to feed on plants.[6]
Thorax

The thorax contains much of the insects means of locomotion, such as the legs and the wings. The thorax consists of three invisibly divided segments, namely the prothorax, metathorax and mesothorax.[8] Adults have two pairs of membranous wings covered, usually completely, by minute scales. In some species, wings are reduced or absent (often in the female but not the male). The Trichoptera (caddisflies) which are a sister group of the Lepidoptera have scales, but also possess caudal cerci on the abdomen, a feature absent in the Lepidoptera.[2]
Abdomen
The abdomen comprises about 9 segments, from segment 5 to 13 in larvae. The eleventh segment of the caterpillar's abdomen holds the anal clasps which are represented as the genitalia which protrudes in the case of some taxa.[8]
Internal physiology
The digestive system of Lepidoptera consists of the proboscis, leading to the esophagus or gullet, and the stomach, over which is a large, bladder-like vessel called the proventriculus, a sort of crop preceding the true stomach, which is a cylindrical tube; the intestine is a slender tube (varying in shape in different genera) divided into the small intestine, the colon, and the rectum.[11]
Polymorphism
Polymorphism is appearance of forms or "morphs" differing in color and number of attributes within a single species.[6]:163[12] In Lepidoptera, polymorphism can be seen not only between individuals in a population, but also between the sexes, termed as sexual dimorphism, between geographically separated populations in geographical polymorphism and also between generations flying at different seasons of the year (seasonal polymorphism). It also includes the phenomenon of mimicry when mimetic morphs fly alongside non-mimetic morphs in a population of a particular species. Polymorphism occurs both at specific level with heritable variation in the overall morphological design of individuals as well as in certain specific morphological or physiological traits within a species.[6]
Genetic polymorphism

Genetic polymorphism occurs when the morphs are a result of genetic determination only. The extreme case of genetic polymorphism is that of the papilionid Great Mormon (Papilio memnon), where four male forms and many as twenty-six female forms are reported. This species, and others in its genus, have been extensively studied for understanding the genetic basis for polymorphism and Batesian mimicry.[13] [14][15]
In the case of the Scarlet Tiger Moth Callimorpha (Panaxia) dominula (family Arctiidae), which is a diurnal moth occurs in continental Europe, western Asia and southern England, three forms occur in England : the typical homozygote; the rare homozygote (bimacula) and the heterozygote (medionigra). It was studied there by E.B. Ford, and later by P.M. Sheppard and their co-workers over many years. Data is available from 1939 to the present day, got by the usual field method of capture-mark-release-recapture and by genetic analysis from breeding in captivity. The records cover gene frequency and population-size for much of the twentieth century.[16] In this instance the genetics appears to be simple: two alleles at a single locus, producing the three phenotypes. Total captures over 26 years 1939-64 came to 15,784 homozygous dominula (i.e. typica), 1,221 heterozygous medionigra and 28 homozygous bimacula. Now, assuming equal viability of the genotypes 1,209 heterozygotes would be expected, so the field results do not suggest any heterozygous advantage. It was Sheppard who found that the polymorphism is maintained by selective mating: each genotype preferentially mates with other morphs.[17] This is sufficient to maintain the system despite the fact that in this case the heterozygote has slightly lower viability.[18]
Sexual dimorphism

Sexual dimorphism is where differences occur between males and females in a species. In Lepidoptera, sexual dimorphism is widespread and almost completely determined by genetic determination.[19]:163 Sexual dimorphism is present in all families of the Papilionoidoea and more prominent in the Lycaenidae, Pieridae and certain taxa of the Nymphalidae. Apart from color variation which may differ from slight to completely different color-pattern combinations, secondary sexual characteristics may also be present.[20]:25 Different genotypes maintained by natural selection may also be expressed at the same time.[19]:163 Polymorphic and/or mimetic females occur in the case of some taxa in the Papilionidae primarily to obtain a level of protection not available to the male of their species.
The most distinct case of sexual dimorphism is that of adult females of many Psychidae species who have only vestigial wings, legs, and mouthparts as compared to the adult males who are strong fliers with well-developed wings and feathery antennae.[21]
Geographical polymorphism
Geographical polymorphism is where geographical isolation causes a divergence of a species into different morphs. A good example is the Indian White Admiral Limenitis procris which has five forms, each geographically separated from the other by large mountain ranges.[20]:26 An even more dramatic showcase of geographical polymorphism is the Apollo butterfly (Parnassius apollo). Due to the Apollos living in smal local populations, having no contact with each other, but because of the strong stenotopic species and weak migration ability interbreeding between populations of one species practically does not occur; they form over 600 different morphs, with the size of spots on the wings of which varies greatly.[22]
Environmental polymorphism
_in_Kawal_WS,_AP_W_IMG_1784.jpg)

Environmental polymorphism, where genetic heritability plays no role, is often termed as polyphenism. Polyphenism in Lepidoptera is commonly seen in the form of seasonal morphs especially in the butterfly families of Nymphalidae and Pieridae. The Old World pierid butterfly, the Common Grass Yellow (Eurema hecabe) has a darker summer adult morph, triggered by a long day exceeding 13 hours in duration, while the shorter diurnal period of 12 hours or less induces a fairer morph in the post-monsoon period.[19]:164 Polyphenism also occurs in caterpillars, an example being the American Peppered Moth Biston betularia (shown below).[23]
Mimicry
Batesian and Müllerian mimicry complexes are commonly found in Lepidoptera. Genetic polymorphism and natural selection give rise to other-wise edible species (the mimic) gaining a survival advantage by resembling inedible Lepidoptera species (the model). Such a mimicry complex is referred to as Batesian and is most commonly known by the mimicry by the limenitidine Viceroy butterfly of the inedible danaine Monarch. Later research has discovered that the Viceroy is, in fact more toxic than the Monarch and this resemblance should be considered as a case of Müllerian mimicry.[24]
In Müllerian mimicry, inedible species, usually within a taxonomic order, find it advantageous to resemble each other so as to reduce the sampling rate by predators who need to learn about the insects' inedibility. Taxa from the toxic genus Heliconius form one of the most well Müllerian complexes known.[25] The adults of the various species now resemble each other so well that the species cannot be distinguished without close morphological observation and, in some cases, dissection and/or genetic analysis.
Reproduction and development
Species of Lepidoptera undergo Holometabolism, a form of metamorphism called complete metamorphism. Their life cycle normally consist of an egg, larva, pupa, and an imago or adult.[6] The larvae are commonly called caterpillars, and the pupa of moths called cocoons and of butterflies called chrysalis.
Mating
Mating would begin where an adult (female or male) would attract a mate normally using visual stimuli, specially in diurnal species like most butterflies. However, most nocturnal female species (e.g., moths) use pheromones instead to attract males, even sometimes from over long distances.[6] Some species engage in a form of acoustic courtship, or attract mates using sound or vibration such as the Polka-dot wasp moth (Syntomeida epilais).[26]
Life cycle


Lepidopteran usually reproduce sexually and are oviparous (egg-laying), though some species give to live birth in a process called ovoviviparity. There is a variety of difference in egg-laying and the number of eggs laid. Some species simply drop their eggs in flight (these species normally have polyphagous larvae, or eat a variety of plants e.g., Hepialids and some Nymphalids[27]) while most Lepidopteran will lay their eggs near or on the host plant that larva feed on, normally attracted by its odor. The amount of eggs laid may vary from only a few to thousands.[6]
The larvae, or first stage in their life cycle after hatching, look very different from the adults and come in a variety of shapes and sizes. However, they are characterized by an elongated body with 0–11 abdominal legs (usually 8) and hooklets, called apical crochets, towards a well developed head with mandibles.[6] The larvae eat every part of the plant, and are normally considered pest to their host plant; species have been found to lay their eggs on the fruit and other species lay the eggs even on clothing or fur (e.g., Clothing Moths). A species of Geometridae from Hawaii has carnivorous larvae that grab and eat flies.[4] Some species are carnivorous and others are even parasitic. The larvae develop rapidly with several generations in a year, however some species may take up to 3 years to develop.[6]
After about 5 to 7 instars,[28]:26–28 or molts, regulated by certain hormones like prothoracicotropic hormone stimulates the production of ecdysone, telling the insect to start molting. Then, the larva puparium, a sclerotized or hardened cuticle of the last larval instar, develops into the pupa. The pupa may be covered in silk and attached with many different types of debris or nothing at all depending on the species. The time it takes for pupae to emerge will vary between species. The adult will emerge from the pupa either by using abdominal hooks or a projection from the head.[6]
While most Lepidoptera are terrestrial, many species of Pyralidae are truly aquatic with all stages except the adult occurring in water. Many species from other families such as Arctiidae, Nepticulidae, Cosmopterygidae, Tortricidae, Olethreutidae, Noctuidae, Cossidae and Sphingidae are aquatic or semi-aquatic.[29]:22
Behavior and ecology
Flight
The main form of locomotion in most Lepidoptera species is flight. In some species, there is sometimes a gliding component to their flight. Flight occurs either as hovering, or as forward or backward motion.[30]

Navigation is important to Lepidoptera species, specially for those that migrate. Butterflies, who have more species that migrate, have been shown to navigate using time compensated sun compasses. They can see polarized light and therefore orient even in cloudy conditions. The polarized light in the region close to the ultraviolet spectrum is suggested to be particularly important.[31] It is suggested that most migratory butterflies are those that belong to semi-arid areas where breeding seasons are short.[32] The life-histories of their host plants also influence the strategies of the butterflies.[33] Other theories include the use of landscapes. Lepidoptera may use coastal lines, mountains, but also man-made roads to orient themselves. Above sea it has been observed that the flight direction is much more accurate if the landscape on the coast is still visible.[34]
Moths also show navigation, as seen in many studies. One study showed that many moths may use Earth's magnetic field to navigate, as a study of the stray Heart and Dart suggests.[35] Another study, this time of the migratory behavior of the Silver Y, showed that this species, even at high altitudes, can correct its course with changing winds, and prefers flying with favourable winds, which suggests a great sense of direction.[36][37] Aphrissa statira in Panama loses its navigational capacity when exposed to a magnetic field, suggesting it uses the Earth’s magnetic field.[38]
Moths exhibit a tendency to circle artificial lights repeatedly. This suggests that these species use a technique of celestial navigation called transverse orientation. By maintaining a constant angular relationship to a bright celestial light, such as the Moon, they can fly in a straight line. Celestial objects are so far away, that even after traveling great distances, the change in angle between the moth and the light source is negligible; further, the moon will always be in the upper part of the visual field or on the horizon. When a moth encounters a much closer artificial light and uses it for navigation, the angle changes noticeably after only a short distance, in addition to being often below the horizon. The moth instinctively attempts to correct by turning toward the light, causing airborne moths to come plummeting downwards, and - at close range - which results in a spiral flight path that gets closer and closer to the light source.[39]
Other explanations have been suggested, such as the idea that moths may be impaired with a visual distortion called a Mach band by Henry Hsiao in 1972. He stated that they fly towards the darkest part of the sky in pursuit of safety and are thus inclined to circle ambient objects in the Mach band region.[40]
Migration

Lepidopteran migration is usually seasonal, moving to escape dry seasons or other disadvantageous conditions. Most lepidopteran that migrate are butterflies, varying from short to over long distances. Some butterflies that migrate include the Mourning Cloak, Painted Lady, American Lady, Red Admiral, and the Common Buckeye.[28]:29–30 Particularly famous migrations are those of the Monarch butterfly from Mexico to northern USA and southern Canada, a distance of about 4000 to 4800 km (2500–3000 mi). Other well known migratory species include the Painted Lady and several of the Danaine butterflies. Spectacular and large scale migrations associated with the Monsoons are seen in peninsular India.[41] Migrations have been studied in more recent times using wing tags and also using stable hydrogen isotopes.[42][43]
Moths also undergo migrations, such as the uraniids. U. fulgens undergoes population explosions and massive migrations that may be not surpassed by any other insect in the Neotropics. In Costa Rica and Panama, the first population movements may begin in July and early August and, depending on the year, may be very massive, continuing unabated for as long as five months.[44]
Communication
Pheromones are commonly involved in mating rituals amongst species, especially moths, but pheromones are an important aspect of other forms of communication amongst species as well. Usually only one sex will produce the pheromones and the other would pick them up with its antennae.[37] In many female species, a gland between the eighth and ninth segment under the abdomen produces the pheromones.[6]
Communication can also occur through stridulation, or producing sounds by rubbing various parts of the body together.[37] Some species engage in a form of acoustic courtship, or attract mates using sound or vibration such as the Polka-dot wasp moth (Syntomeida epilais).[26]
Defense and predation
Lepidopterans are soft bodied and move slowly, therefore at risk to predators including birds, wasps, and mammals. Some caterpillars, such as the zebra swallowtail butterfly larvae, are cannibalistic and may eat other larvae of the same species. Wasps and flies may lay eggs in the caterpillar which would eventually kill it as they hatch inside its body and eat its tissues. Butterflies are more fragile and almost defenseless. Lepidopterans rely on a variety of strategies for defense and protection.[45]
.jpg)
Some species of lepidoptera are poisonous to predators, such as the monarch butterfly and pipevine swallowtail butterfly. They obtain their toxicity from the plants they eat. The brightly colored caterpillars and adults are generally the toxic ones, giving their color a reminder to predators about their toxicity. Predators that eat poisonous lepidopterans may become sick and vomit violently, learning not to eat those types of lepidopterans. A predator who has previously eaten a poisonous lepidopteran may avoid other species with similar markings in the future, thus saving many other species as well.[45][46]
Other caterpillars emit bad smells to ward off predators.[45] Some caterpillars, especially members of Papilionidae, contain an osmeterium, a Y-shape protrusible gland found in the prothoracic segment of the larvae. When threatened, the caterpillar emits unpleasant smells from the organ to ward off the predators.[47][48]
Camouflage and mimicry are also important defense strategies. Some lepidopterans blend with its surroundings, making them difficult to be spotted by predators. Caterpillars can be shades of green that matches its host plant. Others look like inedible objects, such as the Western Tiger Swallowtail larvae that look like bird droppings.[45][49] For example, adult Sesiidae species (also known as clearwing moths) have a general appearance that is sufficiently similar to a wasp or hornet to make it likely that the moths gain a reduction in predation by Batesian mimicry.[50]
Eyespots are a type of automimicry used by some lepidopterans. In butterflies, the spots are composed of concentric rings of scales of different colors. Studies have investigated their role in defensive behavior. The proposed role of the eyespots is to deflect attention to predators. Their resemblance to eyes provokes the predator's instinct to attack these wing patterns.[51]
Evolution
Not much is known about ancient Lepidoptera species because so few fossils have been found. The earliest known lepidopteran fossil, Archaeolepis mane is from the Jurassic period, about 190 million years ago. The fossil consists of a pair of wings with scales that are characteristically similar to the wing venation pattern found in Trichoptera (caddisflies). 2 other sets of Jurassic Lepidopteran fossils have been found, and 13 sets from the Cretaceous period.[52] The best preserved fossil lepidopteran is the Eocene Prodryas persephone from the Florissant Fossil Beds.
Phylogeny
|
Lepidoptera and Trichoptera (caddisflies) share many similarities that are lacking in other insect orders.
- The females of both orders are heterogametic, meaning they have two different sex chromosomes, whereas in most species the males are heterogametic and the females have two identical sex chromosomes.
- Adults in both orders display a particular wing venation pattern on their forewings.
- The larvae of both orders have mouth structures and gland with which they make and manipulate silk.[52]
Willi Hennig grouped the two sister orders into the Amphiesmenoptera superorder. This group probably evolved in the Jurassic, having split from the now extinct order Necrotaulidae.[52]
Micropterigidae, Agathiphagidae and Heterobathmiidae are the oldest and most basal lineages of Lepidoptera. The adults of these families do not have the curled tongue or proboscis that are found in most members order, but instead have chewing mandibles adapted for a special diet. Micropterigidae larvae feed on leaves, fungi, or liverworts (much like the Trichoptera).[7] Adult Micropterigidae chew the pollen or spores of ferns. In the Agathiphagidae, larvae live inside kauri pines and feed on seeds. In Heterobathmiidae the larvae feed on the leaves of Nothofagus, the southern beech tree. These families also have mandibles in the pupal stage, which help the pupa emerge from the seed or cocoon after metamorphosis.[7]
The Eriocraniidae have a short coiled proboscis in the adult stage, and though they retain their pupal mandibles with which they escaped the cocoon, their mandibles are non-functional thereafter.[7] Most of these non-ditrysian families, are primarily leaf miners in the larval stage. In addition to the proboscis, there is a change in the scales among these basal lineages, with later lineages showing more complex perforated scales.[52]
With the evolution of the Ditrysia in the mid-Cretaceous, there was a major reproductive change. The Ditrysia, which comprise 98% of the Lepidoptera, have two separate openings for reproduction in the females (as well as a third opening for excretion), one for mating, and one for laying eggs. The two are linked internally by a seminal duct. (In more basal lineages there is one cloaca, or later, two openings and an external sperm canal.) Of the early lineages of Ditrysia, Gracillarioidea and Gelechioidea are mostly leaf miners, but more recent lineages feed externally. In the Tineoidea, most species feed on plant and animal detritus and fungi, and build shelters in the larval stage.[52]
The Yponomeutoidea is the first group to have significant numbers of species whose larvae feed on herbaceous plants, as opposed to woody plants.[52] They evolved about the time that flowering plants underwent an expansive adaptive radiation in the mid-Cretaceous, and the Gelechioidea that evolved at this time also have great diversity. Whether the processes involved co-evolution or sequential evolution, the diversity of the Lepidoptera and the angiosperms increased together.
In the so-called "macrolepidoptera", which constitutes about 60% of Lepidopteran species, there was a general increase in size, better flying ability (via changes in wing shape and linkage of the forewings and hindwings), reduction in the adult mandibles, and a change in the arrangement of the crochets (hooks) on the larval prolegs, perhaps to improve the grip on the host plant.[52] Many also have tympanal organs, that allow them to hear. These organs evolved eight times, at least, because they occur on different body parts and have structural differences.[52] The main lineages in the macrolepidoptera are the Noctuoidea, Bombycoidea, Lasiocampidae, Mimallonoidea, Geometroidea and Rhopalocera. Bombycoidea plus Lasiocampidae plus Mimallonoidea may be a monophyletic group.[52] The Rhopalocera, comprising the Papilionoidea (Butterflies), Hesperioidea (skippers), and the Hedyloidea (moth-butterflies), are the most recently evolved.[7] There is quite a good fossil record for this group, with the oldest skipper about 56 million years old.[52]
Taxonomy and systematics
Distinguishing characteristics
The characteristics which distinguish the order Lepidoptera from other insect orders are:[54]
- Head: The Lepidopteran head has large compound eyes and mouth parts which are almost always a proboscis.
- Scales: Scales cover the external surface of the body and appendages.
- Thorax: The prothorax in the case of most species is reduced.
- Wings: Two pairs of wings present in almost the taxa. The wings have very few cross-veins.
- Abdomen: The posterior abdominal segments are modified extensively for reproduction. Cerci are absent.
- Larva: The larvae are eruciform with well developed head and mandibles. They have 0 to 10 prolegs, usually 8.
- Pupa: The pupae in most species are adecticous and obtect, while they are decticous in others.
History of study
Linnaeus in Systema Naturae (1758) recognized three divisions of the Lepidoptera: Papilio, Sphinx, and Phalaena with seven subgroups in Phalaena.[7] These persist today as 9 of the superfamilies of Lepidoptera. Other works on classification followed including those by Denis & Ignaz Schiffermüller (1775), Fabricius (1775) and Pierre André Latreille (1796). Jacob Hübner described many genera, and the Lepidopteran genera were catalogued by Ochsenheimer and Treitschke in a series of volumes on the Lepidopteran fauna of Europe published between 1807 and 1835.[7] G.A.W. Herrich-Schaffer (several volumes, 1843–1856), and Edward Meyrick (1895) based their classifications primarily on wing venation. Sir George Francis Hampson worked on the 'microlepidoptera' during this period and Philipp Christoph Zeller published The Natural History of the Tineinae13 volumes also on 'microlepidoptera'(1855).
Among the first entomologists to study fossil insects and their evolution was Samuel Hubbard Scudder (1837–1911), who worked on butterflies.[52] He published a study of the Florissant deposits of Colorado. Andreas V. Martynov (1879–1938) recognized the close relationship between Lepidoptera and Trichoptera in his studies on phylogeny.[52] Lepidoptera tend not to be as common as some other insects in the habitats that are most conducive to fossilization, such as lakes and ponds, and their juvenile stage has only the head capsule as a hard part that might be preserved. Yet there are fossils, some preserved in amber and some in very fine sediments. Leaf mines are also seen in fossil leaves, although the interpretation of them is tricky.[52] The earliest fossil is Archaeolepis mane from the Jurassic, about 190 million years ago in Dorset, UK.[52] It consists of wings and shows scales with parallel grooves under a scanning electron microscope and the characteristic wing venation pattern shared with Trichoptera.[52] Only 2 more sets of Jurassic Lepidopteran fossils have been found, and 13 sets in the Cretaceous.[52] From there, many more fossils are found from the Tertiary, and particularly the Eocene Baltic amber.
Major contributions in the 20th century included the creation of the monotrysia and ditrysia (based on female genital structure) by Borner in 1925 and 1939.[7] Willi Hennig (1913–1976) developed the cladistic methodology and applied it to insect phylogeny. Niels P. Kristensen, E. S. Nielsen and D. R. Davis studied the relationships among monotrysian families and Kristensen worked more generally on insect phylogeny and higher Lepidoptera too.[7][52]. While it is often found that DNA-based phylogenies differ from those based on morphology, this has not been the case for the Lepidoptera; DNA phylogenies correspond to a large extent to morphology-based phylogenies.[52]
Many attempts have been made to group the superfamilies of the Lepidoptera into natural groups, most of which fail because one of the two groups is not monophyletic: Microlepidotera and Macrolepidoptera, Heterocera and Rhopalocera, Jugatae and Frenatae, Monotrysia and Ditrysia.[7]
Relationship to people
In culture


Artistic depictions of butterflies have been used in many cultures including as early as 3500 years ago, in Egyptian hieroglyphs.[55] Today, butterflies are widely used in various objects in art and jewelry: mounted in frames, embedded in resin, displayed in bottles, laminated in paper, and in some mixed media artworks and furnishings.[56] Butterflies have also inspired the "butterfly fairy" as an art and fictional character, including in the Barbie Mariposa film.
In many cultures the soul of a dead person is associated with the butterfly. As in Ancient Greece, where the word for butterfly ψυχή (psyche) also means soul and breath. In Latin, as in Ancient Greece, the word for "butterfly" papillio was associated with the soul of the dead.[57]
The Death's-head Hawkmoth's skull pattern on its thorax has helped these moths, particularly A. atropos, earn a negative reputation, such as associations with the supernatural and evil. The moth has been prominently featured in art and movies such as Un Chien Andalou (by Buñuel and Dalí) and The Silence of the Lambs, and in the artwork of the Japanese metal band Sigh's Hail Horror Hail album.
As pests
The larvae of many Lepidopteran species are major pests in agriculture. Some of the major pests include Tortricidae, Noctuidae, and Pyralidae. The larvae of the Noctuidae genus Spodoptera (armyworms) and Helicoverpa (corn earworm) can cause extensive damage to certain crops.[7] Helicoverpa zea larvae are polyphagous, meaning they eat a variety of crops. Other common crops consumed are tomatoes, by the tomato fruitworm, and cotton, by cotton bollworms.[58]
As beneficial
Most species of Lepidoptera engage in the pollination of flowers.[59] The adults feed on the nectar inside flowers, using their proboscis to reach the nectar hidden at the base of the petals. In the process, the adult brushes against the flower's stamen, on which the flower's reproductive pollen is made and stored. The pollen is transferred to the adult, who flies to the next flower to feed and unwittingly deposits the pollen on the stigma of the next flower, where the pollen germinates and fertilizes the seeds.
The larvae of Bombyx mori are more commonly known as silkworms. They make their cocoons out of silk which can be spun into cloth. Silk is and has been an important economic resource throughout history. The species Bombyx mori has been domesticated to the point where it is completely dependent on humans for survival.[60] On the other hand, the species Bombyx mandarina, or "Wild Silkmoth," lives and produces silk naturally.[61]
As food

Lepidoptera feature prominently in entomophagy as food items on almost every continent. While in most cases, adults, larvae or pupae are eaten as staples by indigenous people, beondegi or silkworm pupae are eaten as a snack in Korean cuisine[62] while Maguey worm is considered a delicacy in Mexico.[63] In the Carnia region of Italy, children catch and eat Zygaena moths in early summer. The ingluvies, despite having a very low cyanogenic content, serves as a convenient, supplementary source of sugar to the children who can include this resource as a seasonal delicacy at minimum risk.[64]
Butterfly ranching
Butterfly ranching in Papua New Guinea permits nationals of that country to 'farm' economically valuable insect species for the collectors market in an ecologically sustainable manner.[65]
See also
- McGuire Center for Lepidoptera and Biodiversity, University of Florida
- Difference between a butterfly and a moth
- Moth
- List of moths
- Butterfly
- List of butterflies in Taiwan
- List of butterflies of Great Britain
- List of butterflies of Tobago
- List of butterflies of Menorca
- List of butterflies of India
- List of butterflies of North America
- Societas Europaea Lepidopterologica
References
- ↑ "The Lepidoptera Taxome Project Draft Proposals and Information". Centre for Ecology and Evolution, University College London. http://www.ucl.ac.uk/taxome/. Retrieved 2007-03-05.
- ↑ 2.0 2.1 Resh, V. H.; R. T. Cardé (Editor) (2003). "Lepidoptera". Encyclopedia of Insects. Jerry A. Powell (Editor of Section). Academic Press. pp. 631–664. http://www.scribd.com/doc/13060600/Encyclopedia-of-Insects-Arab2000Forumprofr.
- ↑ Capinera, John L. (2008). "Butterflies and moths". Encyclopedia of Entomology. 4 (2 ed.). Dept. of Entomology and Nematology, UF: Gainesville, Fl: Springer Science+Business Media B.V.. pp. 626–672. ISBN 978140206242. http://books.google.com/?id=i9ITMiiohVQC&printsec=frontcover#v=onepage&q=Butterflies%20and%20Moths%20(Lepidoptera). Retrieved 03 Oct 2009.
- ↑ 4.0 4.1 Arnett, Ross H. (July 28, 2000). "Part I: 27". American insects: a handbook of the insects of America north of Mexico (2 ed.). CRC Press. pp. 631. ISBN 0849302129. http://books.google.com/?id=4M0v0Ye54MYC&printsec=frontcover&q=leidoptera.
- ↑ 5.0 5.1 Harpe, Douglas; Dan McCormack (November 2001). "Online Etymological Dictionary". Online Etymological Dictionary. LogoBee.com. pp. 1. http://www.etymonline.com/. Retrieved 6 December 2009.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 Gullan, P. J.; P. S. Cranston (September 13, 2004). "7". The insects: an outline of entomology (3 ed.). Wiley-Blackwell. pp. 198–199. ISBN 1405111135. http://books.google.com/?id=qHtMPvaAfKIC&printsec=frontcover&dq=entomology&q=Lepidoptera%20larva.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 Scoble, Malcolm J. (September 1995). "2". The Lepidoptera: Form, Function and Diversity (1 ed.). Oxford University: Oxford University Press. pp. 4–5. ISBN 0198549520. http://books.google.com/?id=gnpd_5iNTiwC&printsec=frontcover&q=.
- ↑ 8.0 8.1 8.2 8.3 Evans, W.H. (December 31, 1931). "Introduction". Identification of Indian Butterflies (2 ed.). Madras: Bombey Natural History Society. pp. 1 to 35. ISBN 9780785530473. http://books.google.com/?id=2DdHAAAACAAJ&dq=Identification+of+Indian+Butterflies.
- ↑ Merlin, Christine; Robert J. Gegear, Steven M. Reppert (25 September 2009). "Antennal Circadian Clocks Coordinate Sun Compass Orientation in Migratory Monarch Butterflies". Science 325 (5948): 1700–1704. doi:10.1126/science.1176221. PMID 19779201.
- ↑ Scoble, M.J. (1995). "The Adult Head - Feeding and Sensation". The Lepidoptera: form, function and diversity. Oxford University, USA: Oxford University Press. pp. 4 to 22. ISBN 0198540310. http://books.google.com/?id=gnpd_5iNTiwC&lpg=PA1&dq=Lepidoptera%20morphology&pg=PA63#v=onepage&q=head. Retrieved 04 Oct 2009.
- ↑ Holland, W. J. (January 1, 1907). The Butterfly Book. A Popular Guide to a Knowledge of the Butterflies of North America. New York: Doubleday, Page & Co.. pp. 22–23. http://books.google.com/?id=SyZDAAAAYAAJ&pg=PA22&dq=Lepidoptera+digestive+system#v=onepage&q=.
- ↑ Ford E.B. Genetic polymorphism. Oxford. p11
- ↑ Clarke, C.A.; Sheppard, P.M. & Thornton, I.W.B. The Genetics of the Mimetic Butterfly Papilio Memnon L. Philosophical Transactions of the Royal Society, London. (B - Biological Sciences) 22 August 1968 vol. 254 no. 791 37-89. Abstract. Accessed on 20 Jan 2010.
- ↑ Clarke, C.A. & Sheppard, P.M. - Further Studies on the Genetics of the Mimetic Butterfly Papilio memnon L. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, Vol. 263, No. 847 (Oct. 7, 1971), pp. 35-70. Abstract. Accessed on 20 Jan 2010.
- ↑ Clarke, C.A. & Sheppard, P.M. The Genetics of Four New Forms of the Mimetic Butterfly Papilio memnon L. Proceedings of the Royal Society of London. Series B, Biological Sciences, Vol. 184, No. 1074 (Aug. 31, 1973), pp. 1-14. Abstract. Accessed on 20 Jan 2010.
- ↑ Ford E.B. 1971. Ecological genetics. 3rd ed London 1971, chapter7.
- ↑ Sheppard P.M. 1952. A note on non-random mating in the moth Panaxia dominula (L.). Heredity 6: 239-41.
- ↑ Sheppard P.M. and Cook L.M. 1962. The manifold effects of the medionigra gene in the moth Panaxia dominula and the maintenance of polymorphism. Heredity 17:415-26.
- ↑ 19.0 19.1 19.2 Gullan & Cranston (2005). Sec 6.8 Polymorphism and polyphenism.
- ↑ 20.0 20.1 Kunte, Krushnamegh.(2000).Butterflies of Peninsular India. Part of Project lifescape.Orient Blackswan. ISBN 8173713545, ISBN 9788173713545.
- ↑ "Psychidae at Bug Guide". Iowa State University. http://bugguide.net/node/view/122. Retrieved 2010-01-19.
- ↑ Ivy IG, Morgun DV, Dovgailo KE, Rubin, NI, Solodovnikov IA Дневные бабочки (Hesperioidea and Papilionoidea, Lepidoptera) Восточной Европы." CD determinant, database and software package «Lysandra». - Minsk, Kiev, Moscow: 2005. In Russian
- ↑ Noor MA, Parnell RS, Grant BS (2008). "A Reversible Color Polyphenism in American Peppered Moth (Biston betularia cognataria) Caterpillars". PLoS ONE 3 (9): e3142. doi:10.1371/journal.pone.0003142. PMID 18769543. PMC 2518955. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0003142.
- ↑ Ritland, D.; L. P. Brower (1991). "The viceroy butterfly is not a Batesian mimic". Nature 350: 497–498. doi:10.1038/350497a0. http://www.nature.com/nature/journal/v350/n6318/abs/350497a0.html. Retrieved 2008-02-23. "Viceroys are as unpalatable as monarchs, and significantly more unpalatable than queens from representative Florida populations.".
- ↑ Meyer A (2006) Repeating Patterns of Mimicry. PLoS Biol 4(10): e341 doi:10.1371/journal.pbio.0040341
- ↑ 26.0 26.1 Sanderford, M. V.; W. E. Conner (July, 1990). "Courtship sounds of the polka-dot wasp moth,Syntomeida epilais". Naturwissenschaften (Wake Forest University, North Carolina, USA: Springer Berlin / Heidelberg) 77 (Volume 77, Number 7 / July, 1990): 345–347. doi:10.1007/BF01138395. OCLC 0028-1042. http://www.springerlink.com/content/t74276m35731224r/fulltext.pdf?page=1.
- ↑ Wiklund, Christer (July, 1984). "Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants". Oecologia (Springer Berlin / Heidelberg) 63 (Volume 63, Number 1 / July, 1984): 23–29. doi:10.1007/BF00379780. OCLC 0029-8549. http://www.springerlink.com/content/rk558621g45r0636/fulltext.pdf?page=1.
- ↑ 28.0 28.1 Dole, Claire Hagen (May 28, 2003). The Butterfly Gardener's Guide. Brooklyn Botanic Garden. ISBN 1889538582. http://books.google.com/?id=e69Q6J2i1qwC&pg=PA26&dq=metamorphosis+butterfly&cd=2#v=onepage&q=metamorphosis%20butterfly.
- ↑ Ward, JV & Peter. Insct Ecology.
- ↑ Scoble, Malcolm (1995-07-01). The Lepidoptera: Form, Function and Diversity. Oxford University Press, 1995. pp. 66–67. ISBN 0198549520. http://books.google.com/?id=gnpd_5iNTiwC&pg=PA66&dq=Lepidoptera+flight&cd=4#v=onepage&q=Lepidoptera%20flight.
- ↑ Sauman, Ivo; Adriana D. Briscoe, Haisun Zhu, Dingding Shi, Oren Froy, Julia Stalleicken, Quan Yuan, Amy Casselman, and Steven M. Reppert (May 5, 2005). "Connecting the Navigational Clock to Sun Compass Input in Monarch Butterfly Brain". Neuron (Neuron) 46 (3): 457–467. doi:10.1016/j.neuron.2005.03.014. PMID 15882645. http://www.cell.com/neuron/abstract/S0896-6273(05)00236-9.
- ↑ Southwood, T. R. E. (1962). "Migration of terrestrial arthropods in relation to habitat.". Biological Reviews (Cambridge Philosophical Society) 37 (2): 171–211. doi:10.1111/j.1469-185X.1962.tb01609.x. http://www3.interscience.wiley.com/journal/119869297/abstract.
- ↑ Dennis, Roger L. H.; Tim G. Shreeve, Henry R. Arnold, and David B. Roy (September, 2005). "Does diet breadth control herbivorous insect distribution size? Life history and resource outlets for specialist butterflies". Journal of Insect Conservation (Springer Netherlands) 9 (3): 187–200. doi:10.1007/s10841-005-5660-x. http://www.springerlink.com/content/g712p32787l5g6qh/fulltext.pdf.
- ↑ Made, J.G. van der; Josef Blab, Rudi Holzberger, H. van den Bijtel (1989) (in Dutch). Actie voor Vlinders, zo kunnen we ze redden.. Weert: M & P cop.. pp. 192. ISBN 9065903038.
- ↑ Baker, R. Robin (February 1987). "Integrated use of moon and magnetic compasses by the heart-and-dart moth, Agrotis exclamationis". Animal Behaviour 35 (1): 94–101. doi:10.1016/S0003-3472(87)80214-2. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W9W-4JT84SP-F&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=1129652847&_rerunOrigin=scholar.google&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=2892ea838fdde22df54095b4505c1c2b.
- ↑ Breen, Amanda (May 7, 2008). "Scientists make compass discovery in migrating moths". University of Greenwich at Medway. pp. 1. http://www.gre.ac.uk/pr/articles/2008news/a1537---moths. Retrieved 9 December 2009.
- ↑ 37.0 37.1 37.2 Chapman, Jason W.; Don R. Reynolds, Henrik Mouritsen, Jane K. Hill, Joe R. Riley, Duncan Sivell, Alan D. Smith, and Ian P. Woiwod (8 April 2008). "Wind selection and drift compensation optimize migratory pathways in a high-flying moth". Current Biology 18 (7): 514–518. doi:10.1016/j.cub.2008.02.080. PMID 18394893. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6VRT-4S6GG6K-7-7&_cdi=6243&_user=10&_coverDate=04%2F08%2F2008&_sk=%23TOC%236243%232008%23999819992%23684615%23FLA%23display%23Volume_18,_Issue_7,_Pages_471-552_(8_April_2008)%23tagged%23Volume%23first%3D18%23Issue%23first%3D7%23date%23(8_April_2008)%23&view=c&_gw=y&wchp=dGLbVlz-zSkWb&md5=dfc8a24f69a6b84eef8f82cf4394d837&ie=/sdarticle.pdf.
- ↑ Srygley, Robert B.; Evandro G. Oliveira, Andre J . Riveros (2005). "Experimental evidence for a magnetic sense in Neotropical migrating butterflies (Lepidoptera: Pieridae)". The British Journal of Animal Behaviour 71 (1): 183–191. doi:10.1016/j.anbehav.2005.04.013. ISSN 0003-3472. http://users.ox.ac.uk/~zool0206/AnimBeh06.pdf.
- ↑ Elliot, Debbie; Dr. May Berenbaum (August 18, 2007). "Why are Moths Attracted to Flame? (audio)". National Public Radio. pp. 1. http://www.npr.org/templates/story/story.php?storyId=12903572. Retrieved 12 December 2009.
- ↑ Hsiao, Henry S. (1972). Attraction of moths to light and to infrared radiation. San Francisco Press. ISBN 0911302212.
- ↑ Williams, C. B. 1927 A study of butterfly migration in south India and Ceylon, based largely on records by Messrs. G Evershed, E.E.Green, J.C.F. Fryer and W. Ormiston. Trans. Ent. Soc. London 75:1-33
- ↑ Urquhart, F. A. & N. R. Urquhart. 1977. Overwintering areas and migratory routes of the Monarch butterfly (Danaus p. plexippus, Lepidoptera: Danaidae) in North America, with special reference to the western population. Can. Ent. 109: 1583-1589
- ↑ Wassenaar L.I., Hobson K.A. 1998. Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence. Proc Natl Acad Sci U S A. 95(26):15436-9. Full text
- ↑ Smith, N. G.; Janzen, D.H. (editor) (1983b). Urania fulgens (Calipato Verde, Green Urania).. Costa Rican Natural History. Chicago: University of Chicago Press. pp. 816.
- ↑ 45.0 45.1 45.2 45.3 "Caterpillar and Butterfly Defense Mechanisms". EnchantedLearning.com. http://www.enchantedlearning.com/subjects/butterfly/allabout/Defense.shtml. Retrieved 7 December 2009.
- ↑ Kricher, John (1999-08-16). "6". A Niotropical Companion. Princeton University Press. pp. 157–158. ISBN 9780691009742. http://books.google.com/?id=Z3pgdvrSmG8C&pg=PA158&dq=defense+protection+butterflies&cd=7#v=onepage&q=defense%20protection%20butterflies.
- ↑ "osmeterium". Merriam-Webster, Incorporated. http://www.merriam-webster.com/dictionary/osmeterium. Retrieved 9 December 2009.
- ↑ Hadley, Debbie. "Osmeterium". About.com Guide. http://insects.about.com/od/entomologyglossary/g/def_osmeterium.htm. Retrieved 9 December 2009.
- ↑ Latimer, Jonathan P.; Karen Stray Nolting (May 30, 2000). Butterflies. Houghton Mifflin Harcourt Trade & Reference Publis. pp. 12. ISBN 0395979447. http://books.google.com/?id=5P-oyg5Dgl8C&pg=PA12&dq=Tiger+swallowtail&cd=1#v=onepage&q=Tiger%20swallowtail.
- ↑ Corporation, Marshall Cavendish (January 2003). Insects and Spiders of the World,. 10. Marshall Cavendish Corporation. Marshall Cavendish Inc. pp. 292–293. ISBN 0761473440. http://books.google.com/?id=uvdpiSQUuesC&pg=PA592&dq=clearwing+moths+mimicry&cd=2#v=onepage&q=.
- ↑ Carroll, Sean (2005). Endless forms most beautiful: the new science of evo devo and the making of the animal kingdom. W.W. Norton & Co.. pp. 205–210. ISBN 0393060160. http://books.google.com/?id=CnnGKjzw3xMC&pg=PA205&dq=Butterfly+eyespots+defense&cd=3#v=onepage&q=Butterfly%20eyespots%20defense.
- ↑ 52.00 52.01 52.02 52.03 52.04 52.05 52.06 52.07 52.08 52.09 52.10 52.11 52.12 52.13 52.14 52.15 52.16 52.17 Grimaldi, D. and Engel, M.S. (2005). Evolution of the Insects. Cambridge University Press. ISBN 0-521-82149-5.
- ↑ "Ditrysia". Tree of Life Web Project. The Tree of Life Web Project. 1 May 2008. pp. 1.
- ↑ Gillot, C. Entomology 2nd Ed. (1995) Springer, ISBN 0306449676, 9780306449673. Accessed on Google Books on 25 Nov 2009.
- ↑ Larsen, Torben B. (1994). Online "Butterflies of Egypt". Saudi Aramco world (Saudi Aramco world) 45 (5): 24–27. http://www.saudiaramcoworld.com/issue/199405/butterflies.of.egypt.htm Online. Retrieved 2009-12-18.
- ↑ "Table complete with real butterflies embedded in resin". Mfjoe.com. 2009-12-18. http://mfjoe.com/tag/furniture/. Retrieved 2009-03-30.
- ↑ Rabuzzi, Matthew (November 1997). "Butterfly Etymology Cultural Entomology Digest 4". Cupertino, California: Bugbios. p. 4. http://www.insects.org/ced4/etymology.html. Retrieved 2009-12-18.
- ↑ Cook, Kelly A.; Weinzier, R. (2004). "IPM: Field Crops: Corn Earworm (Heliothis Zea)". IPM. pp. 1. http://www.ipm.uiuc.edu/fieldcrops/insects/corn_earworm/index.html. Retrieved January 17, 2009.
- ↑ Gilbert LE (1972). "Pollen feeding and reproductive biology of Heliconius butterflies". Proceedings of the National Academy of Sciences 69: 1402–1407. doi:10.1073/pnas.69.6.1403. http://www.pnas.org/content/69/6/1403.abstract.
- ↑ Goldsmith MR, Shimada T, Abe H. (2005). The genetics and genomics of the silkworm, Bombyx mori. Annu Rev Entomol. 50:71-100. PMID 15355234
- ↑ Yoshitake, N. (1968). "Phylogenetic aspects on the origin of Japanese race of the silkworm, Bombyx mori". Journal of Sericological Sciences of Japan 37: 83–87.
- ↑ Martin Robinson, Ray Bartlett, Rob Whyte. Korea (2007). Lonely Planet publications, ISBN1741045584, ISBN 9781741045581. (pg 63)
- ↑ http://www.insectia.com/beta/e/dr_c2508724.html
- ↑ http://www.bioone.org/doi/abs/10.2993/0278-0771-29.1.64
- ↑ http://www.butterfliesandart.com/Butterfly_Farms/Butterfly_Farms.htm
Further reading
- Kristensen, N.P. (Ed.). 1999. Lepidoptera, Moths and Butterflies. Volume 1: Evolution, Systematics, and Biogeography. Handbuch der Zoologie. Eine Naturgeschichte der Stämme des Tierreiches / Handbook of Zoology. A Natural History of the phyla of the Animal Kingdom. Band / Volume IV Arthropoda: Insecta Teilband / Part 35: 491 pp. Walter de Gruyter, Berlin, New York.
- Nye, I.W.Bb & Fletcher,D.S. 1991. Generic Names of Moths of the World. Volume 6: xxix + 368 pp. Trustees of the British Museum (Natural History), London.
- O'Toole, Christopher. 2002. Firefly Encyclopedia of Insects and Spiders. ISBN 1-55297-612-2.
- Nemos, F. Europas bekannteste Schmetterlinge. Beschreibung der wichtigsten Arten und Anleitung zur Kenntnis und zum Sammeln der Schmetterlinge und Raupen. Oestergaard Verlag, Berlin, ca. 1895, http://epic.awi.de/Publications/Dem1895a.pdf (pdf, 77 MB).
- Walsh, P.M., Boyd,T., Nash, D.W., Rolston, E. and Tyner, A. 2009. Report on migrant and notable Lepidoptera in Ireland, 2006. Ir. Nat J. 30: 40 - 50.
External links
- European Butterflies and Moths by Christopher Jonko
- Norwegian Butterflies and Moths (Huge picture archive)
- Moths and Butterflies of Europe and North Africa
- British Butterflies and Moths
- Butterflies of Bulgaria
- Photography of European Butterflies and Moths
- Butterflies and Moths in the Netherlands
- Swedish Moths and Butterflies - Lepidoptera.se
- Butterflies of Asturias - Spain
- Lepidoptera of French Antilles
- Butterflies of Asian Russia
- Butterflies from Indo_China
- Butterflies of Turkey
- Moths of Jamaica
- Historic Moth illustrations
- "Lepidoptera". Integrated Taxonomic Information System. http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=117232.
- Caught Between the Pages: Treasures from the Franclemont Collection Online virtual exhibit featuring a selection of historic entomological writings and images from the Comstock Library of Entomology at Cornell University
- Japmoth Japanese moths. Access images via the numbers on the left.
- Literaturatenbank Free downloads
|
|||||||||||||||||||||||||||||||