Lactose
| Lactose(Milk sugar) | |
|---|---|
 |
|
|
β-D-galactopyranosyl-(1→4)-D-glucose
|
|
|
Other names
Milk sugar
4-O-β-D-galactopyranosyl-D-glucose |
|
| Identifiers | |
| CAS number | 63-42-3 |
| PubChem | 6134 |
| EC-number | 200-559-2 |
|
SMILES
O[C@H]2[C@H](O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)[C@@H](CO)O[C@@H](O)[C@@H]2O
|
|
| Properties | |
| Molecular formula | C12H22O11 |
| Molar mass | 342.30 g/mol |
| Appearance | white solid |
| Solubility in water | 21.6 g/100 mL |
| Hazards | |
| EU Index | not listed |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Lactose is a sugar that is found most notably in milk and is formed from galactose and glucose. Lactose makes up around 2~8% of milk (by weight), although the amount varies among species and individuals. It is extracted from sweet or sour whey. The name comes from lac, the Latin word for milk, plus the -ose ending used to name sugars. It has a formula of C12H22O11.
Contents |
History
Lactose was discovered in milk in 1619 by Fabriccio Bartoletti, and identified as a sugar in 1780 by Carl Wilhelm Scheele.[1]
Chemistry
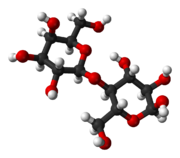
Lactose is a disaccharide that consists of galactose and glucose fragments bonded through a β-1→4 glycosidic linkage. Its systematic name is β-D-galactopyranosyl-(1→4)-D-glucose. The glucose fragment can be in either the α-pyranose form or the β-pyranose form, whereas the galactose fragment can only have the β-pyranose form: hence α-lactose and β-lactose refer to anomeric form of the glucopyranose ring alone.
As it gives free radicals by mechanochemistry, it is possible to use lactose to follow by electron spin resonance(ESR) the energy used during a milling process.[2]
Lactose is hydrolysed to glucose and galactose, isomerised in alkaline solution to lactulose, and catalyticaly hydrogenated to the corresponding polyhydric alcohol, lactitol.[1]
Solubility
The solubility of lactose in water is 18.9049 g at 25 °C, 25.1484 g at 40 °C and 37.2149 g at 60 °C per 100 g solution. Its solubility in ethanol is 0.0111 g at 40 °C and 0.0270 g at 60 °C per 100 g solution.[3] ds
Catabolism
Infant mammals nurse on their mothers to drink milk, which is rich in the carbohydrate lactose. The intestinal villi secrete an enzyme called lactase (β-D-galactosidase) to digest it. This enzyme cleaves the lactose molecule into its two subunits, the simple sugars glucose and galactose, which can be absorbed.
Since lactose occurs mostly in milk, in most mammals the production of lactase gradually decreases with maturity due to a lack of constant consumption.
Many people with ancestry in Europe, West Asia, India, and parts of East Africa maintain lactase production into adulthood. In many of these areas, milk from mammals such as cattle, goats, and sheep is used as a large source of food. Hence, it was in these regions that genes for lifelong lactase production first evolved. The genes of lactose tolerance have evolved independently in various ethnic groups.[4] By descent, more than 70% of western Europeans can drink milk as adults, compared with less than 30% of people from areas of Africa, eastern and south-eastern Asia and Oceania.[5]
People who are lactose intolerant may suffer uncomfortable or socially unacceptable symptoms of too much lactose consumption. In these people, lactose is not broken down and provides food for gas-producing gut flora. This can lead to bloating, flatulence, and other gastrointestinal symptoms.
Lactose can also be bought in pure form, as an assist in high calorie diets.[6]
Industrial production and usage
It has been estimated that the annual worldwide availability of lactose as a by-product of the dairy industry is several million tons. Whey contains about 4.8% of lactose, which may be purified by crystallisation. Food industry applications, both of pure lactose and lactose-containing dairy by-products, have markedly increased since the 1960s. For example, its bland flavour has lent to its use as a carrier and stabiliser of aromas and pharmaceutical products.
Lactose is little fermented by baker's yeast and during brewing, which may be used to advantage.[1] Lactose is sometimes used in stout beers to sweeten the beer and is non-fermentable in beer.
See also
- Sugars in wine
References
- ↑ 1.0 1.1 1.2 Linko, P (1982), "Lactose and Lactitol", in Birch, G.G. & Parker, K.J, Nutritive Sweeteners, London & New Jersey: Applied Science Publishers, pp. 109–132, ISBN 0-85334-997-5
- ↑ Baron, M.; Chamayou, A.; Marchioro, L.; Raffi, J. (2005), "Radicalar probes to measure the action of energy on granular materials", Adv. Powder Technol. 16 (3): 199–212, doi:10.1163/1568552053750242.
- ↑ Machado, José J. B.; Coutinho, João A.; Macedo, Eugénia A. (2001), "Solid–liquid equilibrium of α-lactose in ethanol/water", Fluid Phase Equilibria 173 (1): 121–34, doi:10.1016/S0378-3812(00)00388-5, http://path.web.ua.pt/file/FPE%20(2000)%20173%20121.pdf.
- ↑ Wade, Nicholas (2006-12-10), "Study Detects Recent Instance of Human Evolution", New York Times, http://www.nytimes.com/2006/12/10/science/10cnd-evolve.html?.
- ↑ Ridley, Matt (1999), Genome, HarperCollins, p. 193, ISBN 978-0-06-089408-5.
- ↑ Cooper, Lenna F.; Edith M Barber, Helen S Mitchell (1947), Nutrition in Health and Disease (10th ed.), J.B. Lipincott, p. 414
|
|||||||||||||||||||||||||||||||||||||||||||||