Tryptophan
| L-Tryptophan | |
|---|---|
 |
|
 |
|
|
Tryptophan or (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid
|
|
|
Other names
2-Amino-3-(1H-indol-3-yl)propanoic acid
|
|
| Identifiers | |
| CAS number | 73-22-3 |
| PubChem | 6305 |
| IUPHAR ligand | 717 |
| ATC code | N06 |
|
SMILES
N[C@@H](Cc1c2ccccc2n([H])c1)C(O)=O
|
|
| Properties | |
| Molecular formula | C11H12N2O2 |
| Molar mass | 204.23 g mol−1 |
| Solubility in water | Soluble: 0.23 g/L at 0 °C, 11.4 g/L at 25 °C, |
| Solubility | Soluble in hot alcohol, alkali hydroxides; insoluble in chloroform. |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Tryptophan (IUPAC-IUBMB abbreviation: Trp or W; IUPAC abbreviation: L-Trp or D-Trp; sold for medical use as Tryptan)[1] is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG. Twiptophan can be used as a mnemonic for the single letter code W.[2] Only the L-stereoisomer of tryptophan is used in structural or enzyme proteins, but the D-stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan).[3] The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as defined by its growth effects on rats.
Contents |
Isolation
The isolation of tryptophan was first reported by Frederick Hopkins in 1901[4] through hydrolysis of casein. From 600 grams of crude casein one obtains 4-8 grams of tryptophan.[5]
Biosynthesis and industrial production
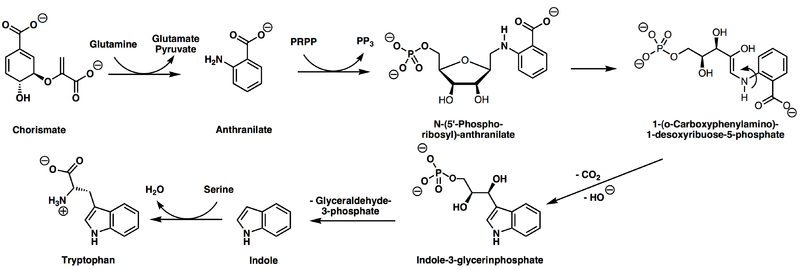
Plants and microorganisms commonly synthesize tryptophan from shikimic acid or anthranilate.[6] The latter condenses with phosphoribosylpyrophosphate (PRPP), generating pyrophosphate as a by-product. After ring opening of the ribose moiety and following reductive decarboxylation, indole-3-glycerinephosphate is produced, which in turn is transformed into indole. In the last step, tryptophan synthase catalyzes the formation of tryptophan from indole and the amino acid serine.
The industrial production of tryptophan is also biosynthetic and is based on the fermentation of serine and indole using either wild-type or genetically modified bacteria such as Corynebacterium glutamicum, Bacillus subtilis, Bacillus amyloliquefaciens or E. coli. These strains carry either mutations that prevent the reuptake of aromatic amino acids or multiple/overexpressed trp operons. The conversion is catalyzed by the enzyme tryptophan synthase.[7]
Function
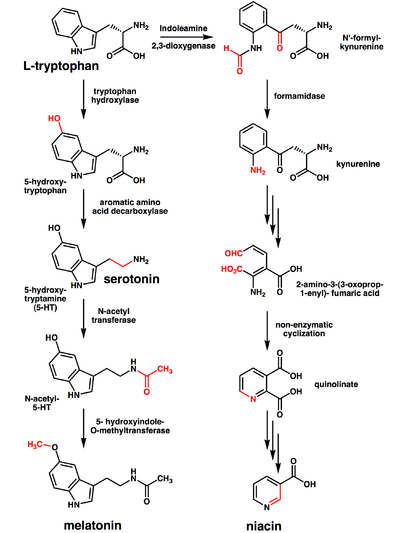
For many organisms (including humans), tryptophan is an essential amino acid. This means that it cannot be synthesized by the organism and therefore must be part of its diet. Amino acids, including tryptophan, act as building blocks in protein biosynthesis. In addition, tryptophan functions as a biochemical precursor for the following compounds (see also figure to the right):
- Serotonin (a neurotransmitter), synthesized via tryptophan hydroxylase.[8][9] Serotonin, in turn, can be converted to melatonin (a neurohormone), via N-acetyltransferase and 5-hydroxyindole-O-methyltransferase activities.[10]
- Niacin is synthesized from tryptophan via kynurenine and quinolinic acids as key biosynthetic intermediates.[11]
- Auxin (a phytohormone) when sieve tube elements undergo apoptosis tryptophan is converted to auxins.[12]
The disorders fructose malabsorption and lactose intolerance causes improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood[13] and depression.[14]
In bacteria that synthesize tryptophan, high cellular levels of this amino acid activate a repressor protein, which binds to the trp operon.[15] Binding of this repressor to the tryptophan operon prevents transcription of downstream DNA that codes for the enzymes involved in the biosynthesis of tryptophan. So high levels of tryptophan prevent tryptophan synthesis through a negative feedback loop and, when the cell's tryptophan levels are reduced, transcription from the trp operon resumes. The genetic organisation of the trp operon thus permits tightly regulated and rapid responses to changes in the cell's internal and external tryptophan levels.
Dietary sources
Tryptophan is a routine constituent of most protein-based foods or dietary proteins. It is particularly plentiful in chocolate, oats, dried dates, milk, yogurt, cottage cheese, red meat, eggs, fish, poultry, sesame, chickpeas, sunflower seeds, pumpkin seeds, spirulina, and peanuts.[16] Despite popular belief to the contrary, the level found in turkey is at a level typical of poultry in general.[17]
| Food | Protein [g/100 g of food] |
Tryptophan [g/100 g of food] |
Tryptophan/Protein [%] |
|---|---|---|---|
| egg, white, dried |
|
|
|
| spirulina, dried |
|
|
|
| cod, atlantic, dried |
|
|
|
| soybeans, raw |
|
|
|
| cheese, Parmesan |
|
|
|
| caribou |
|
|
|
| sesame seed |
|
|
|
| cheese, cheddar |
|
|
|
| sunflower seed |
|
|
|
| pork, chop |
|
|
|
| turkey |
|
|
|
| chicken |
|
|
|
| beef |
|
|
|
| salmon |
|
|
|
| lamb, chop |
|
|
|
| perch, Atlantic |
|
|
|
| egg |
|
|
|
| wheat flour, white |
|
|
|
| baking chocolate, unsweetened |
|
|
|
| milk |
|
|
|
| rice, white |
|
|
|
| oatmeal, cooked |
|
|
|
| potatoes, russet |
|
|
|
| banana |
|
|
|
Use as a dietary supplement
For some time, tryptophan has been available in health food stores as a dietary supplement. Many people found tryptophan to be a safe and reasonably effective sleep aid, probably due to its ability to increase brain levels of serotonin (a calming neurotransmitter when present in moderate levels)[19] and/or melatonin (a sleep-inducing hormone secreted by the pineal gland in response to darkness or low light levels).[20][21]
Clinical research has shown mixed results with respect to tryptophan's effectiveness as a sleep aid, especially in normal patients.[22][23][24] Furthermore tryptophan has shown some effectiveness for treatment of a variety of other conditions typically associated with low serotonin levels in the brain[25] such as premenstrual dysphoric disorder[26] and seasonal affective disorder.[27][28] In particular, tryptophan has shown considerable promise as an antidepressant alone[29] and as an "augmenter" of antidepressant drugs.[29][30] However, the reliability of these clinical trials has been questioned.[31][32]
Metabolites
A metabolite of tryptophan, 5-Hydroxytryptophan (5-HTP), has been suggested as a treatment for epilepsy[33] and depression, although clinical trials are regarded inconclusive and lacking.[34]
Due to the conversion of 5-HTP into serotonin by the liver, there may be a significant risk of heart valve disease from serotonin's effect on the heart.[35][36] In Europe, 5-HTP is prescribed with carbidopa to prevent the conversion of 5-HTP into serotonin until it reaches the brain.[37]
Since 5-HTP readily crosses the blood-brain barrier and in addition is rapidly decarboxylated to serotonin (5-hydroxytryptamine or 5-HT),[38] it may be useful for the treatment of depression. However, serotonin has a relatively short half-life since it is rapidly metabolized by monoamine oxidase, and therefore is likely to have limited efficacy. It is marketed in Europe for depression and other indications under the brand names Cincofarm, Tript-OH and Optimax (UK).
In the United States, 5-HTP does not require a prescription, as it is covered under the Dietary Supplement Act. Since the quality of dietary supplements is now regulated by the U.S. Food and Drug Administration there is now a guarantee that the label accurately depicts what the bottle contains.[39]
As 5-HTP is usually converted to serotonin before it can reach the brain, elevating blood serotonin levels greatly, it may cause diarrhea and heart problems, while only slightly increasing brain serotonin. Therefore, 5-HTP is more effectively used when in conjunction with a dopa decarboxylase inhibitor such as Carbidopa, which slows its conversion to serotonin, allowing more of supplement to reach the brain.
Tryptophan supplements and EMS
Although now available for purchase, there was a large tryptophan-related outbreak of eosinophilia-myalgia syndrome (EMS) in 1989 which caused 1,500 cases of permanent disability and at least thirty-seven deaths. Some epidemiological studies[40][41][42] traced the outbreak to L-tryptophan supplied by a Japanese manufacturer, Showa Denko KK.[43] It was further hypothesized that one or more trace impurities produced during the manufacture of tryptophan may have been responsible for the EMS outbreak.[44][45] The fact that the Showa Denko facility used genetically engineered bacteria to produce L-tryptophan gave rise to speculation that genetic engineering was responsible for such impurities.[46][47] However, the methodology used in the initial epidemiological studies has been criticized.[48][49] An alternative explanation for the 1989 EMS outbreak is that large doses of tryptophan produce metabolites which inhibit the normal degradation of histamine and excess histamine in turn has been proposed to cause EMS.[50]
Most tryptophan was banned from sale in the US in 1991, and other countries followed suit. Tryptophan from one manufacturer, of six, continued to be sold for manufacture of baby formulas. At the time of the ban, the FDA did not know, or did not indicate, that EMS was caused by a contaminated batch,[51][52] and yet, even when the contamination was discovered and the purification process fixed, the FDA maintained that L-tryptophan was unsafe. In February 2001, the FDA loosened the restrictions on marketing (though not on importation), but still expressed the following concern:
- "Based on the scientific evidence that is available at the present time, we cannot determine with certainty that the occurrence of EMS in susceptible persons consuming L-tryptophan supplements derives from the content of L-tryptophan, an impurity contained in the L-tryptophan, or a combination of the two in association with other, as yet unknown, external factors."[43]
Since 2002, L-tryptophan has been sold in the U.S. in its original form. Several high-quality sources of L-tryptophan do exist, and are sold in many of the largest health food stores nationwide. Indeed, tryptophan has continued to be used in clinical and experimental studies employing human patients and subjects.
In recent years in the U.S., compounding pharmacies and some mail-order supplement retailers have begun selling tryptophan to the general public. Tryptophan has also remained on the market as a prescription drug (Tryptan), which some psychiatrists continue to prescribe, particularly as an augmenting agent for people who are unresponsive to antidepressant drugs.
Turkey meat and drowsiness
One belief is that heavy consumption of turkey meat (as for example in a Thanksgiving or Christmas feast) results in drowsiness, which has been attributed to high levels of tryptophan contained in turkey.[53][54][55] While turkey does contain high levels of tryptophan, the amount is comparable to that contained in most other meats.[17]
Furthermore, post-meal drowsiness on Thanksgiving may have more to do with what else is consumed along with the turkey, in particular carbohydrates and alcohol. It has been demonstrated in both animal models[56] and in humans[57][58][59] that ingestion of a meal rich in carbohydrates triggers release of insulin. Insulin in turn stimulates the uptake of large neutral branched-chain amino acids (LNAA) but not tryptophan (trp) into muscle, increasing the ratio of trp to LNAA in the blood stream. The resulting increased ratio of tryptophan to large neutral amino acids in the blood reduces competition at the large neutral amino acid transporter resulting in the uptake of tryptophan across the blood-brain barrier into the central nervous system (CNS).[60][61] Once inside the CNS, tryptophan is converted into serotonin in the raphe nuclei by the normal enzymatic pathway.[56][58] The resultant serotonin is further metabolised into melatonin by the pineal gland.[10] Hence, these data suggest that "feast-induced drowsiness," and in particular, the common post-Christmas and North American post-Thanksgiving dinner drowsiness, may be the result of a heavy meal rich in carbohydrates which, via an indirect mechanism, increases the production of sleep-promoting melatonin in the brain.[56][57][58][59]
Fluorescence
See also
- Serotonin
- 5-HTP
- Tryptamine
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. http://www.chem.qmul.ac.uk/iupac/AminoAcid/. Retrieved 2007-05-17.
- ↑ "Dr. Margaret Oakley Dayhoff". The Chemistry of Amino Acids. University of Arizona. http://www.biology.arizona.edu/biochemistry/problem_sets/aa/Dayhoff.html. Retrieved 7 September 2010.
- ↑ Pallaghy PK, Melnikova AP, Jimenez EC, Olivera BM, Norton RS (1999). "Solution structure of contryphan-R, a naturally-occurring disulfide-bridged octapeptide containing D-tryptophan: comparison with protein loops". Biochemistry 38 (35): 11553–9. doi:10.1021/bi990685j. PMID 10471307.
- ↑ Hopkienns FG, Cole SW (1901). "A contribution to the chemistry of proteids: Part I. A preliminary study of a hitherto undescribed product of tryptic digestion". J. Physiol. (Lond.) 27 (4-5): 418–28. PMID 16992614. PMC 1540554. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1540554.
- ↑ Cox GJ, King H (1943), "L-Tryptophane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0612; Coll. Vol. 2: 612–616
- ↑ Radwanski ER, Last RL (1995). "Tryptophan biosynthesis and metabolism: biochemical and molecular genetics". Plant Cell 7 (7): 921–34. doi:10.1105/tpc.7.7.921. PMID 7640526.
- ↑ Ikeda M (2002). "Amino acid production processes". Adv. Biochem. Eng. Biotechnol. 79: 1–35. PMID 12523387. http://www.springerlink.com/content/226q8plt36351kck.
- ↑ Fernstrom JD (1983). "Role of precursor availability in control of monoamine biosynthesis in brain". Physiol. Rev. 63 (2): 484–546. PMID 6132421. http://physrev.physiology.org/cgi/reprint/63/2/484.
- ↑ Schaechter JD, Wurtman RJ (1990). "Serotonin release varies with brain tryptophan levels". Brain Res. 532 (1-2): 203–10. doi:10.1016/0006-8993(90)91761-5. PMID 1704290. http://wurtmanlab.mit.edu/publications/pdf/790.pdf.
- ↑ 10.0 10.1 Wurtman RJ, Anton-Tay F (1969). "The mammalian pineal as a neuroendocrine transducer". Recent Prog. Horm. Res. 25: 493–522. PMID 4391290. http://wurtmanlab.mit.edu/publications/pdf/104.pdf.
- ↑ Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O (1965). "Studies on the biosynthesis of nicotinamide adenine dinucleotide. II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals". J. Biol. Chem. 240: 1395–401. PMID 14284754. http://www.jbc.org/cgi/reprint/240/3/1395.
- ↑ Palme K, Nagy F (April 2008). "A new gene for auxin synthesis". Cell 133 (1): 31–2. doi:10.1016/j.cell.2008.03.014. PMID 18394986.
- ↑ Ledochowski M, Widner B, Murr C, Sperner-Unterweger B, Fuchs D (2001). "Fructose malabsorption is associated with decreased plasma tryptophan". Scand. J. Gastroenterol. 36 (4): 367–71. doi:10.1080/003655201300051135. PMID 11336160.
- ↑ Ledochowski M, Sperner-Unterweger B, Widner B, Fuchs D (1998). "Fructose malabsorption is associated with early signs of mental depression". Eur. J. Med. Res. 3 (6): 295–8. PMID 9620891.
- ↑ Gollnick P, Babitzke P, Antson A, Yanofsky C (2005). "Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis". Annu. Rev. Genet. 39: 47–68. doi:10.1146/annurev.genet.39.073003.093745. PMID 16285852.
- ↑ Tryptophan background
- ↑ 17.0 17.1 17.2 Joanne Holden, Nutrient Data Laboratory, Agricultural Research Service. "USDA National Nutrient Database for Standard Reference, Release 22". United States Department of Agriculture. http://www.ars.usda.gov/ba/bhnrc/ndl. Retrieved 2009-11-29.
- ↑ Rambali B, Andel I van, Schenk E, Wolterink G, Werken G van de, Stevenson H, Vleeming W (2002). "[The contribution of cocoa additive to cigarette smoking addiction"] (PDF). RIVM (report 650270002/2002). http://rivm.nl/bibliotheek/rapporten/650270002.pdf.- The National Institute for Public Health and the Environment (Netherlands)
- ↑ Wurtman RJ, Hefti F, Melamed E (1980). "Precursor control of neurotransmitter synthesis". Pharmacol. Rev. 32 (4): 315–35. PMID 6115400. http://wurtmanlab.mit.edu/publications/pdf/466.pdf.
- ↑ Wurtman RJ, Larin F, Axelrod J, Shein HM, Rosasco K (1968). "Formation of melatonin and 5-hydroxyindole acetic acid from 14C-tryptophan by rat pineal glands in organ culture". Nature 217 (5132): 953–4. doi:10.1038/217953a0. PMID 5300432.
- ↑ Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA (2006). "Tryptophan metabolism in the central nervous system: medical implications". Expert reviews in molecular medicine 8 (20): 1–27. doi:10.1017/S1462399406000068. PMID 16942634.
- ↑ Hartmann E (1982). "Effects of L-tryptophan on sleepiness and on sleep". Journal of psychiatric research 17 (2): 107–13. doi:10.1016/0022-3956(82)90012-7. PMID 6764927.
- ↑ Schneider-Helmert D, Spinweber CL (1986). "Evaluation of L-tryptophan for treatment of insomnia: a review". Psychopharmacology (Berl.) 89 (1): 1–7. doi:10.1007/BF00175180. PMID 3090582.
- ↑ Wyatt RJ, Engelman K, Kupfer DJ, Fram DH, Sjoerdsma A, Snyder F. (1970 Oct 24). "Effects of L-tryptophan (a natural sedative) on human sleep". Lancet 1970 Oct 24,2 (7678): 842–6. ISSN 0140-6736. PMID 4097755.
- ↑ "research summary of Dr. Richard Wurtman, MIT". http://web.mit.edu/bcs/people/wurtman.shtml. Retrieved 2007-08-12.
- ↑ Steinberg S, Annable L, Young SN, Liyanage N (1999). "A placebo-controlled clinical trial of L-tryptophan in premenstrual dysphoria". Biol. Psychiatry 45 (3): 313–20. doi:10.1016/S0006-3223(98)00005-5. PMID 10023508.
- ↑ Lam RW, Levitan RD, Tam EM, Yatham LN, Lamoureux S, Zis AP (1997). "L-tryptophan augmentation of light therapy in patients with seasonal affective disorder". Canadian journal of psychiatry. Revue canadienne de psychiatrie 42 (3): 303–6. PMID 9114947. http://ww1.cpa-apc.org:8080/Publications/Archives/CJP/1997/April/apr97_bc1.htm.
- ↑ Jepson TL, Ernst ME, Kelly MW (1999). "Current perspectives on the management of seasonal affective disorder". J Am Pharm Assoc (Wash) 39 (6): 822–9. PMID 10609448.
- ↑ 29.0 29.1 Thomson J, Rankin H, Ashcroft GW, Yates CM, McQueen JK, Cummings SW (1982). "The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo". Psychological medicine 12 (4): 741–51. doi:10.1017/S0033291700049047. PMID 7156248.
- ↑ Levitan RD, Shen JH, Jindal R, Driver HS, Kennedy SH, Shapiro CM (2000). "Preliminary randomized double-blind placebo-controlled trial of tryptophan combined with fluoxetine to treat major depressive disorder: antidepressant and hypnotic effects". Journal of psychiatry & neuroscience : JPN 25 (4): 337–46. PMID 11022398. PMC 1407729. http://www.cma.ca/index.cfm/ci_id/12652/la_id/1.htm.
- ↑ Meyers S (2000). "Use of neurotransmitter precursors for treatment of depression". Alternative medicine review : a journal of clinical therapeutic 5 (1): 64–71. PMID 10696120. http://www.thorne.com/altmedrev/.fulltext/5/1/64.pdf.
- ↑ Shaw K, Turner J, Del Mar C (2002). "Tryptophan and 5-hydroxytryptophan for depression". Cochrane database of systematic reviews (Online) (1): CD003198. doi:10.1002/14651858.CD003198. PMID 11869656.
- ↑ Kostowski W, Bidzinski A, Hauptmann M, Malinowski JE, Jerlicz M, Dymecki J (1978). "Brain serotonin and epileptic seizures in mice: a pharmacological and biochemical study". Pol J Pharmacol Pharm 30 (1): 41–7. PMID 148040.
- ↑ Turner EH, Loftis JM, Blackwell AD (2006). "Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan". Pharmacol Ther 109 (3): 325–38. doi:10.1016/j.pharmthera.2005.06.004. PMID 16023217.
- ↑ Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen U, Waldum H (March 2005). "Long-term serotonin administration induces heart valve disease in rats". Circulation 111 (12): 1517–22. doi:10.1161/01.CIR.0000159356.42064.48. PMID 15781732.
- ↑ Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B (December 2002). "Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells". Am. J. Pathol. 161 (6): 2209–18. PMID 12466135. PMC 1850896. http://ajp.amjpathol.org/cgi/content/abstract/161/6/2209.
- ↑ "WARNING About 5-hydroxytryptophan". Hotlines. Life Extension Magazine. http://www.lef.org/magazine/hotlines3. Retrieved 2009-02-28.
- ↑ Hardebo JE, Owman C (1980). "Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface". Ann NeurolAnn Neurol 8 (1): 1–31. doi:10.1002/ana.410080102. PMID 6105837.
- ↑ http://www.npicenter.com/anm/templates/newsATemp.aspx?articleid=18838&zoneid=2
- ↑ Slutsker L, Hoesly FC, Miller L, Williams LP, Watson JC, Fleming DW (1990). "Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer". JAMA 264 (2): 213–7. doi:10.1001/jama.264.2.213. PMID 2355442.
- ↑ Back EE, Henning KJ, Kallenbach LR, Brix KA, Gunn RA, Melius JM (1993). "Risk factors for developing eosinophilia myalgia syndrome among L-tryptophan users in New York". J. Rheumatol. 20 (4): 666–72. PMID 8496862.
- ↑ Kilbourne EM, Philen RM, Kamb ML, Falk H (1996). "Tryptophan produced by Showa Denko and epidemic eosinophilia-myalgia syndrome". The Journal of rheumatology. Supplement 46: 81–8; discussion 89–91. PMID 8895184.
- ↑ 43.0 43.1 FDA Information Paper on L-tryptophan and 5-hydroxy-L-tryptophan
- ↑ Mayeno AN, Lin F, Foote CS, Loegering DA, Ames MM, Hedberg CW, Gleich GJ (1990). "Characterization of "peak E," a novel amino acid associated with eosinophilia-myalgia syndrome". Science 250 (4988): 1707–8. doi:10.1126/science.2270484. PMID 2270484.
- ↑ Ito J, Hosaki Y, Torigoe Y, Sakimoto K (1992). "Identification of substances formed by decomposition of peak E substance in tryptophan". Food Chem. Toxicol. 30 (1): 71–81. doi:10.1016/0278-6915(92)90139-C. PMID 1544609.
- ↑ Mayeno AN, Gleich GJ (September 1994). "Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale". Trends Biotechnol. 12 (9): 346–52. doi:10.1016/0167-7799(94)90035-3. PMID 7765187.
- ↑ Smith, Jeffrey K. (2007). Genetic roulette: the documented health risks of genetically engineered foods. Yes! Books. ISBN 0-9729665-2-8.
- ↑ Shapiro S (1996). "Epidemiologic studies of the association of L-tryptophan with the eosinophilia-myalgia syndrome: a critique". The Journal of rheumatology. Supplement 46: 44–58; discussion 58–9. PMID 8895181.
- ↑ Horwitz RI, Daniels SR (1996). "Bias or biology: evaluating the epidemiologic studies of L-tryptophan and the eosinophilia-myalgia syndrome". The Journal of rheumatology. Supplement 46: 60–72. PMID 8895182.
- ↑ Smith MJ, Garrett RH (2005). "A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation". Inflamm. Res. 54 (11): 435–50. doi:10.1007/s00011-005-1380-7. PMID 16307217.
- ↑ FDA Tryptophan Recall
- ↑ Raphals P (2000). "Does medical mystery threaten biotech?". Science 250 (4981): 4981. doi:10.1126/science.2237411. PMID 2237411.
- ↑ "About.com: Does Eating Turkey Make You Sleepy?". http://chemistry.about.com/od/holidaysseasons/a/tiredturkey.htm. Retrieved 2007-08-17.
- ↑ "Howstuffworks.com: Is there something in turkey that makes you sleepy?". http://home.howstuffworks.com/question519.htm. Retrieved 2007-08-17.
- ↑ "Chemistry.org: Thanksgiving, Turkey, and Tryptophan". http://www.chemistry.org/portal/a/c/s/1/feature_ent.html?DOC=enthusiasts%5Cent_tryptophan.html. Retrieved 2007-08-17.
- ↑ 56.0 56.1 56.2 Fernstrom JD, Wurtman RJ (1971). "Brain serotonin content: increase following ingestion of carbohydrate diet". Science 174 (13): 1023–5. doi:10.1126/science.174.4013.1023. PMID 5120086.
- ↑ 57.0 57.1 Lyons PM, Truswell AS (1988). "Serotonin precursor influenced by type of carbohydrate meal in healthy adults". Am. J. Clin. Nutr. 47 (3): 433–9. PMID 3279747. http://www.ajcn.org/cgi/reprint/47/3/433.pdf.
- ↑ 58.0 58.1 58.2 Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ (2003). "Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios". Am. J. Clin. Nutr. 77 (1): 128–32. PMID 12499331. http://www.ajcn.org/cgi/content/abstract/77/1/128.
- ↑ 59.0 59.1 Afaghi A, O'Connor H, Chow CM (2007). "High-glycemic-index carbohydrate meals shorten sleep onset". Am. J. Clin. Nutr. 85 (2): 426–30. PMID 17284739. http://www.ajcn.org/cgi/content/full/85/2/426.
- ↑ Pardridge WM, Oldendorf WH (1975). "Kinetic analysis of blood-brain barrier transport of amino acids". Biochim. Biophys. Acta 401 (1): 128–36. doi:10.1016/0005-2736(75)90347-8. PMID 1148286.
- ↑ Maher TJ, Glaeser BS, Wurtman RJ (1984). "Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake". Am. J. Clin. Nutr. 39 (5): 722–9. PMID 6538743.
External links
- "KEGG PATHWAY: Tryptophan metabolism - Homo sapiens". KEGG: Kyoto Encyclopedia of Genes and Genomes. 2006-08-23. http://www.genome.jp/dbget-bin/www_bget?path:hsa00380. Retrieved 2008-04-20.
- G.P. Moss. "Tryptophan Catabolism (early stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/TrpCat1.html. Retrieved 2008-04-20.
- G.P. Moss. "Tryptophan Catabolism (later stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/TrpCat2.html. Retrieved 2008-04-20.
- B Mikkelson, DP Mikkelson (2007-11-22). "Turkey Causes Sleepiness". Urban Legends Reference Pages. Snopes.com. http://www.snopes.com/food/ingredient/turkey.asp. Retrieved 2008-04-20.
- Wood RM, Rilling JK, Sanfey AG, Bhagwagar Z, Rogers RD (2006). "Effects of tryptophan depletion on the performance of an iterated Prisoner's Dilemma game in healthy adults". Neuropsychopharmacology 31 (5): 1075–84. doi:10.1038/sj.npp.1300932. PMID 16407905.
|
||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||