Methionine
| Methionine | |
|---|---|
 |
|
 |
|
|
Methionine
|
|
|
Other names
2-amino-4-(methylthio)butanoic acid
|
|
| Identifiers | |
| Abbreviations | Met, M |
| CAS number | 59-51-8 63-68-3 (L-isomer) 348-67-4 (D-isomer) |
| PubChem | 876 |
| ChemSpider | 853 |
| EC-number | 200-432-1 |
| ATC code | V03,QA05BA90, QG04BA90 |
|
SMILES
CSCC[C@H](N)C(O)=O
|
|
|
InChI
InChI=1/C5H11NO2S/c1-9-3-2-4(6)5(7)8/h4H,2-3,6H2,1H3,(H,7,8)
|
|
| Properties[1] | |
| Molecular formula | C5H11NO2S |
| Molar mass | 149.21 g mol−1 |
| Appearance | White crystalline powder |
| Density | 1.340 g/cm3 |
| Melting point |
281 °C decomp. |
| Solubility in water | Soluble |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
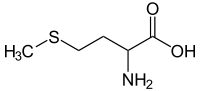
Methionine (pronounced /mɛˈθaɪ.ɵniːn, mɛˈθaɪ.ɵnɪn/; abbreviated as Met or M)[2] is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2SCH3. This essential amino acid is classified as nonpolar.
Contents |
Function
Together with cysteine, methionine is one of two sulfur-containing proteinogenic amino acids. Its derivative S-adenosyl methionine (SAM) serves as a methyl donor. Methionine is an intermediate in the biosynthesis of cysteine, carnitine, taurine, lecithin, phosphatidylcholine, and other phospholipids. Improper conversion of methionine can lead to atherosclerosis.[3]
This amino acid is also used by plants for synthesis of ethylene. The process is known as the Yang Cycle or the methionine cycle.
Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code (tryptophan, encoded by UGG, is the other). The codon AUG is also the "Start" message for a ribosome that signals the initiation of protein translation from mRNA. As a consequence, methionine is incorporated into the N-terminal position of all proteins in eukaryotes and archaea during translation, although it is usually removed by post-translational modification. In bacteria, the derivative N-formylmethionine is used as the initial amino acid.
Betaines

Biosynthesis
As an essential amino acid, methionine is not synthesized in humans, hence we must ingest methionine or methionine-containing proteins. In plants and microorganisms, methionine is synthesized via a pathway that uses both aspartic acid and cysteine. First, aspartic acid is converted via β-aspartyl-semialdehyde into homoserine, introducing the pair of contiguous methylene groups. Homoserine converts to O-succinyl homoserine, which then reacts with cysteine to produce cystathionine, which is cleaved to yield homocysteine. Subsequent methylation of the thiol group by folates affords methionine. Both cystathionine-γ-synthase and cystathionine-β-lyase require pyridoxyl-5'-phosphate as a cofactor, whereas homocysteine methyltransferase requires vitamin B12 as a cofactor.[4]
Enzymes involved in methionine biosynthesis:
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine O-transsuccinylase
- cystathionine-γ-synthase
- cystathionine-β-lyase
- methionine synthase (in mammals, this step is performed by homocysteine methyltransferase)

Other biochemical pathways

Although mammals cannot synthesize methionine, they can still use it in a variety of biochemical pathways:
Generation of homocysteine
Methionine is converted to S-adenosylmethionine (SAM) by (1) methionine adenosyltransferase.
SAM serves as a methyl-donor in many (2) methyltransferase reactions, and is converted to S-adenosylhomocysteine (SAH).
(3) Adenosylhomocysteinase converts SAH to homocysteine.
There are two fates of homocysteine: it can be used to regenerate methionine, or to form cysteine.
Regeneration of methionine
Methionine can be regenerated from homocysteine via (4) methionine synthase.
It can also be remethylated using glycine betaine (NNN-trimethyl glycine) to methionine via the enzyme betaine-homocysteine methyltransferase (E.C.2.1.1.5, BHMT). BHMT makes up to 1.5% of all the soluble protein of the liver, and recent evidence suggests that it may have a greater influence on methionine and homocysteine homeostasis than methionine synthase.
Conversion to cysteine
Homocysteine can be converted to cysteine.
- (5) Cystathionine-β-synthase (a PLP-dependent enzyme) combines homocysteine and serine to produce cystathionine. Instead of degrading cystathionine via cystathionine-β-lyase, as in the biosynthetic pathway, cystathionine is broken down to cysteine and α-ketobutyrate via (6) cystathionine-γ-lyase.
- (7) The enzyme α-ketoacid dehydrogenase converts α-ketobutyrate to propionyl-CoA, which is metabolized to succinyl-CoA in a three-step process (see propionyl-CoA for pathway).
Synthesis
Racemic methionine can be synthesized from diethyl sodium phthalimidomalonate by alkylation with chloroethylmethylsulfide (ClCH2CH2SCH3) followed by hydrolysis and decarboxylation.[5]
Dietary aspects
| Food | g/100g |
|---|---|
| Sesame seeds flour (low fat) | 1.656 |
| Brazil nuts | 1.008 |
| Soy protein concentrate | 0.814 |
| Wheat germ | 0.456 |
| Oat | 0.312 |
| Peanuts | 0.309 |
| Chickpea | 0.253 |
| Corn, yellow | 0.197 |
| Almonds | 0.151 |
| Beans, pinto, cooked | 0.117 |
| Lentils, cooked | 0.077 |
| Rice, brown, medium-grain, cooked | 0.052 |

High levels of methionine can be found in sesame seeds, Brazil nuts, fish, meats and some other plant seeds; methionine is also found in cereal grains. Most fruits and vegetables contain very little of it. Most legumes are also low in methionine. The complement of cereal (methionine) and legumes (lysine), providing a complete protein,[7] is a classic combination, found throughout the world, such as in rice and beans, and similar combinations discussed there.

The use of sesame seeds in cuisine, such as Indian cuisine and, especially in the form of tahini, in Arab cuisine, helps provide essential protein in vegetarian and vegan diets. For example, in hummus, sesame seeds are combined with chickpeas.
Racemic methionine is sometimes added as an ingredient to pet foods.[8]
DL-methionine is the active ingredient in dog supplements to prevent yellow nitrogen burns to grass from their urine. The action is by reducing the pH of the dog's urine.[9] One example is "Grass Saver" by NaturVet.[10] There are claims the supplements can cause bladder stones.[11]
Methionine restriction
There is a growing body of evidence that shows restricting methionine consumption can increase lifespans in some animals.[12]
A 2005 study showed methionine restriction without energy restriction extends mouse lifespan.[13]
A study published in Nature showed adding just the essential amino acid methionine to fruit flies on a calorie restricted diet restored egg-laying without reducing lifespan.[14][15]
See also
- Allantoin
- Formylmethionine
- Paracetamol poisoning#Treatment - A Methionine-Paracetamol preparation that might prevent hepatotoxicity.
- Photo-reactive methionine
References
- ↑ Weast, Robert C., ed. (1981), CRC Handbook of Chemistry and Physics (62nd ed.), Boca Raton, FL: CRC Press, p. C-374, ISBN 0-8493-0462-8.
- ↑ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem. 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ↑ Refsum H, Ueland PM, Nygård O, Vollset SE. Homocysteine and cardiovascular disease. Annual review of medicine, 1998, 49(1), pp.31-62.
- ↑ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000), Principles of Biochemistry (3rd ed.), New York: W. H. Freeman, ISBN 1-57259-153-6.
- ↑ Barger, G.; Weichselbaum, T. E. (1934), "dl-Methionine", Org. Synth. 14: 58, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0384; Coll. Vol. 2: 384.
- ↑ National Nutrient Database for Standard Reference, U.S. Department of Agriculture, http://www.nal.usda.gov/fnic/foodcomp/search/, retrieved 2009-09-07.
- ↑ Nutritional Value – Idaho Bean Commission
- ↑ What's in your dog's food?, Ojibwa Yorkies, ISBN 087605467X, http://www.yorkshire-terrier.com/dogfood.htm, retrieved 2009-09-07.
- ↑ Burn Baby Burn! Grass Burns from Dog Urine, About.Com, http://dogs.about.com/od/dogcarebasics/qt/grass_burns.htm, retrieved 2010-02-15.
- ↑ Grass Saver" by NaturVet, NaturVet, http://www.naturvet.com/index.php?option=com_dogcat&catid=1&subcat=3&Itemid=33.
- ↑ Bladder Stones, Ask.com, http://dogs.about.com/cs/disableddogs/p/bladder_stones.htm.
- ↑ Alleyne, Richard (2009-12-03). "Vegetarian low protein diet could be key to long life". The Daily Telegraph (London). http://www.telegraph.co.uk/health/healthnews/6710896/Vegetarian-low-protein-diet-could-be-key-to-long-life.html. Retrieved 2010-05-12.
- ↑ Miller, Richard A.; Buehner, Gretchen; Chang, Yayi; Harper, James M.; Sigler, Robert; Smith-Wheelock, Michael (2005), "Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance", Aging cell 4 (3): 119–125, doi:10.1111/j.1474-9726.2005.00152.x, PMID 15924568.
- ↑ doi:10.1038/nature08619
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ http://www.sciencenews.org/view/generic/id/50275/title/Amino_acid_recipe_could_be_right_for_long_life
- Rudra, M. N.; Chowdhury, L. M. (30 September 1950), "Methionine Content of Cereals and Legumes", Nature 166 (568): 568, doi:10.1038/166568a0
External links
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||