Kevlar
| Kevlar | |
|---|---|
 |
|
 |
|
| Identifiers | |
| CAS number | 24938-64-5 |
| Properties | |
| Molecular formula | [-CO-C6H4-CO-NH-C6H4-NH-]n |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |

Kevlar is the registered trademark for a para-aramid synthetic fiber, related to other aramids such as Nomex and Technora. Developed at DuPont in 1965,[1][2] this high strength material was first commercially used in the early 1970s as a replacement for steel in racing tires. Typically it is spun into ropes or fabric sheets that can be used as such or as an ingredient in composite material components.
Currently, Kevlar has many applications, ranging from bicycle tires and racing sails to body armor because of its high tensile strength-to-weight ratio—famously: "...5 times stronger than steel on an equal weight basis..."[3] When used as a woven material, it is suitable for mooring lines and other underwater applications.
A similar fiber called Twaron with roughly the same chemical structure was introduced by Akzo in 1978, and now manufactured by Teijin.
Contents |
Production
Kevlar is synthesized in solution from the monomers 1,4-phenylene-diamine (para-phenylenediamine) and terephthaloyl chloride in a condensation reaction yielding hydrochloric acid as a byproduct. The result has liquid-crystalline behavior, and mechanical drawing orients the polymer chains in the fiber's direction. Hexamethylphosphoramide (HMPA) was the solvent initially used for the polymerization, but for safety reasons, DuPont replaced it by a solution of N-methyl-pyrrolidone and calcium chloride. As this process was patented by Akzo (see above) in the production of Twaron, a patent war ensued.
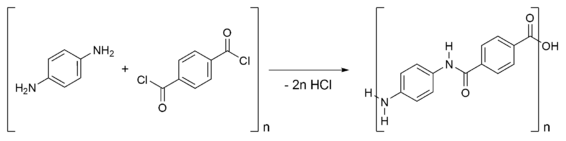
Kevlar (poly paraphenylene terephthalamide) production is expensive because of the difficulties arising from using concentrated sulfuric acid, needed to keep the water-insoluble polymer in solution during its synthesis and spinning.
Three grades of Kevlar are available: (i) Kevlar, (ii) Kevlar 29, and (iii) Kevlar 49. Typically, Kevlar is used as reinforcement in tires and rubber mechanical goods. Kevlar 29's industrial applications are as cables, in asbestos replacement, brake linings, and body armor. Kevlar 49 has a higher strength, and is used in plastic reinforcement for boat hulls, airplanes, and bicycles. The ultraviolet light component of sunlight degrades and decomposes Kevlar, a problem known as UV degradation, and so it is rarely used outdoors without protection against sunlight.
Structure and properties
When Kevlar is spun, the resulting fiber has a tensile strength of about 3 620 MPa, and a relative density of 1.44. The polymer owes its high strength to the many inter-chain bonds. These inter-molecular hydrogen bonds form between the carbonyl groups and NH centers. Additional strength is derived from aromatic stacking interactions between adjacent strands. These interactions have a greater influence on Kevlar than the van der Waals interactions and chain length that typically influence the properties of other synthetic polymers and fibers such as Dyneema. The presence of salts and certain other impurities, especially calcium, could interfere with the strand interactions and caution is used to avoid inclusion in its production. Kevlar's structure consists of relatively rigid molecules which tend to form mostly planar sheet-like structures rather like silk protein.
Thermal properties
Kevlar maintains its strength and resilience down to cryogenic temperatures (-196°C); indeed, it is slightly stronger at low temperatures. At higher temperatures the tensile strength is immediately reduced by about 10-20%, and after some hours the strength progressively reduces further. For example at 160°C about 10% reduction in strength occurs after 500 hours. At 260°C 50% strength reduction occurs after 70 hours.[4]
Applications
Protection
Armor
Kevlar is well-known component of personal armor such as combat helmets, Ballistic face masks, and Ballistic vests. The PASGT helmet and vest used by United States military forces since the early 1980s both have Kevlar as a key component, as do their replacements. Other military uses include bulletproof facemasks used by sentries and spall liners used to protect the crews of armoured fighting vehicles. Related civilian applications include Emergency Service's protection gear if it involves high heat (e.g., tackling a fire), and Kevlar body armor such as vests for police officers, security, and SWAT.
Personal protection
Kevlar is used to manufacture gloves, sleeves, jackets, chaps and other articles of clothing[5] designed to protect users from cuts, abrasions and heat. Kevlar based protective gear is often considerably lighter and thinner than equivalent gear made of more traditional materials.
Sports equipment
It is used as an inner lining for some bicycle tires to prevent punctures, and due to its excellent heat resistance, is used for fire poi wicks. In Table tennis, plies of Kevlar are added to custom ply blades,o paddles, in order to increase bounce and reduce weight. It is used for motorcycle safety clothing, especially in the areas featuring padding such as shoulders and elbows. It was also used as speed control patches for certain Soap Shoes models. In Kyudo or Japanese archery, it may be used as an alternative to more expensive hemp for bow strings. It is one of the main materials used for paraglider suspension lines.
Music
Audio equipment
Kevlar has also been found to have useful acoustic properties for loudspeaker cones, specifically for bass and midrange drive units.[6]
Drumheads
Kevlar is sometimes used as a material on marching snare drums. It allows for an extremely high amount of tension, resulting in a cleaner sound. There is usually some sort of resin poured onto the kevlar to make the head airtight, and a nylon top layer to provide a flat striking surface. This is one of the primary types of marching snare drum heads. Remo's "Falam Slam" Patch is made with kevlar and is used to reinforce bass drum heads where the beater strikes.
Woodwind reeds
Kevlar is used in the woodwind reeds of Fibracell. The material of these reeds is a composite of aerospace materials designed to duplicate the way nature constructs cane reed. Very stiff but sound absorbing Kevlar fibers are suspended in a lightweight resin formulation.
Other uses
Rope, cable, sheath
The fiber is used in woven rope and in cable, where the fibers are kept parallel within a polyethylene sleeve. Known as "Parafil", the cables have been used in small suspension bridges such as the bridge at Aberfeldy in Scotland. They have also been used to stabilise cracking concrete cooling towers by circumferential application followed by tensioning to close the cracks. Kevlar is widely used as a protective outer sheath for optical fiber cable, as its strength protects the cable from damage and kinking.
Electricity generation
Kevlar was used by scientists at Georgia Institute of Technology as a base textile for an experiment in electricity-producing clothing. This was done by weaving zinc oxide nanowires into the fabric. If successful, the new fabric would generate about 80 milliwatts per square meter.[7]
Building construction
A retractable roof of over 60,000 square feet (5,575 square metres) of Kevlar was a key part of the design of Montreal's Olympic stadium for the 1976 Summer Olympics. It was spectacularly unsuccessful, as it was completed ten years late and replaced just ten years later in May 1998 after a series of problems.[8][9]
Brakes
The chopped fiber has been used as a replacement for asbestos in brake pads. Dust produced from asbestos brakes is toxic, while aramids are a benign substitute.
Expansion joints and hoses
Kevlar can be found as a reinforcing layer in rubber bellows expansion joints and rubber hoses, for use in high temperature applications, and for its high strength. It is also found as a braid layer used on the outside of hose assemblies, to add protection against sharp objects.
Particle physics experiment
A thin kevlar window has been used by the NA48 experiment at CERN to separate a vacuum vessel from a vessel at nearly atmospheric pressure, both 192 cm in diameter. The window has provided vacuum tightness combined with reasonably small amount of material (only 0.3% to 0.4% of radiation length).
Composite materials
Aramid fibers are widely used for reinforcing composite materials, often in combination with carbon fiber and glass fiber. The matrix for high performance composites is usually epoxy resin. Typical applications include monocoque bodies for F1 racing cars, helicopter rotor blades, tennis, table tennis, badminton and squash rackets, kayaks, cricket bats, and field hockey, ice hockey and lacrosse sticks.[10][11][12][13]
See also
|
|
References
- ↑ Stephanie Kwolek,Hiroshi Mera and Tadahiko Takata “High-Performance Fibers” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_001
- ↑ "What is Kevlar". DuPont. http://www.dupont.com/kevlar/whatiskevlar.html. Retrieved 2007-03-28.
- ↑ "What is Kevlar". DuPont. http://www.dupont.com/kevlar/whatiskevlar.html. Retrieved 2007-03-28.
- ↑ KEVLAR Technical Guide
- ↑ Kevlar® - DuPont Personal Protection
- ↑ Audio speaker use
- ↑ Scientific American: Fabric Produces Electricity As You Wear It
- ↑ Roof of the Montreal Olympic Stadium at Structurae
- ↑ Clem's Baseball ~ Olympic Stadium
- ↑ Kadolph, Sara J. Anna L. Langford. Textiles, Ninth Edition. Pearson Education, Inc 2002. Upper Saddle River, NJ
- ↑ D. Tanner, J. A. Fitzgerald, B. R. Phillips (1989). "The Kevlar Story - an Advanced Materials Case Study". Angewandte Chemie International Edition in English 28 (5): 649–654. doi:10.1002/anie.198906491.
- ↑ E. E. Magat (1980). "Fibers from Extended Chain Aromatic Polyamides, New Fibers and Their Composites". Philosophical Transactions of the Royal Society of London Series A 294 (1411): 463–472. http://links.jstor.org/sici?sici=0080-4614%2819800121%29294%3A1411%3C463%3AFFECAP%3E2.0.CO%3B2-P.
- ↑ Ronald V. Joven. Manufacturing Kevlar panels by thermo-curing process. Los Andes University, 2007. Bogotá, Colombia.
External links
- Kevlar Home Page
- Aramids
- Kevlar - Design Dictionary. Illustrated article about Kevlar
- Matweb material properties of Kevlar
- U.S. Patent 5,565,264
- Kevlar
- Synthesis of Kevlar
- Aberfeldy Footbridge over the River Tay
|
|||||||||||||||||||||||
|
|||||||||||||||||||||
