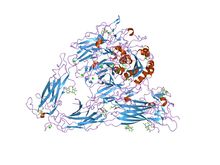
Integrin
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Integrins are receptors that mediate attachment between a cell and the tissues surrounding it, which may be other cells or the extracellular matrix (ECM). They also play a role in cell signaling and thereby define cellular shape, mobility, and regulate the cell cycle.
Typically, receptors inform a cell of the molecules in its environment and the cell evokes a response. Not only do integrins perform this outside-in signalling, but they also operate an inside-out mode. Thus, they transduce information from the ECM to the cell as well as reveal the status of the cell to the outside, allowing rapid and flexible responses to changes in the environment, for example to allow blood coagulation by platelets.
There are many types of integrin, and many cells have multiple types on their surface. Integrins are of vital importance to all animals and have been found in all animals investigated, from sponges to mammals. Integrins have been extensively studied in humans.
Integrins work alongside other proteins such as cadherins, cell adhesion molecules and selectins to mediate cell-cell and cell-matrix interaction and communication. Integrins bind cell surface and ECM components such as fibronectin, vitronectin, collagen, and laminin.
Contents |
Structure
Integrins are obligate heterodimers containing two distinct chains, called the α (alpha) and β (beta) subunits. In mammals, 18 α and 8 β subunits have been characterized, whereas the Drosophila genome encodes only five α and two β subunits, and the Caenorhabditis nematodes possess two α and one β genes.[3] Both the α and β subunits contain two separate tails, both of which penetrate the plasma membrane and possess small cytoplasmic domains.[4]
|
alpha
|
In addition, variants such as of some of the subunits are formed by differential splicing, for example 4 variants of the beta-1 subunit exist. Through different combinations of these alpha and beta subunits, some 24 unique integrins are generated, although the number varies according to different studies.[5]
Integrin subunits span the plasma membrane and in general have very short cytoplasmic domains of about 40–70 amino acids. The exception is the beta-4 subunit which has a cytoplasmic domain of 1088 amino acids, one of the largest known cytoplasmic domains of any membrane protein. Outside the cell plasma membrane, the alpha and beta chains lie close together along a length of about 23 nm, the final 5 nm N-termini of each chain form a ligand-binding region for the ECM, or extracellular matrix.
The molecular mass of the integrin subunits can vary from 90 kDa to 160 kDa. β subunits have four cysteine-rich repeated sequences. Both α and β subunits bind several divalent cations. The role of the α subunit is unknown, but they may stabilize the folds of the protein. The β subunits are more interesting: they are directly involved in coordinating at least some of the ligands that integrins bind.
There are various ways of categorizing the integrins. For example, a subset of the α chains has an additional structural element (or "domain") inserted toward their N-terminal, the so called alpha-A domain (because it has a similar structure to the A-domains found in the protein von Willebrand factor: it is also termed the α-I domain). Integrins carrying this domain either bind to collagens (e.g. integrins α1 β1, and α2 β1), or act as cell-cell adhesion molecules (integrins of the β2 family). This α-I domain is the binding site for ligands of such integrins. Those integrins that don't carry this inserted domain, also have an A-domain in their ligand binding site, but this A-domain is found on the β subunit.
In both cases, the A-domains carry up to three divalent cation binding sites. One is permanently occupied in physiological concentrations of divalent cations, and carries either a calcium or magnesium ion, the principal divalent cations in blood at median concentrations of 1.4 mM (calcium) and 0.8 mM (magnesium). The other two sites become occupied by cations when ligands bind—at least for those ligands involving an acidic amino acid in their interaction sites. An acidic amino acid features in the integrin-interaction site of many ECM proteins, for example, as part of the amino acid sequence Arginine-Glycine-Aspartic acid ("RGD" in the one-letter amino acid code).
High resolution structure
Despite many years of effort, discovering the high resolution structure of integrins proved to be challenging: membrane proteins are classically difficult to purify, and integrins are also large, complex and linked to many sugar trees ("highly glycosylated"). Low resolution images of detergent extracts of intact integrin GPIIbIIIa, obtained using electron microscopy, and even data from indirect techniques, investigating the solution properties of integrins using ultracentrifugation and light scattering, were combined with fragmentary high resolution crystallographic or NMR data from single or paired domains of single integrin chains, and molecular models postulated for the rest of the chains. Despite these wide-ranging efforts, the X-ray crystal structure obtained for the complete extracellular region of one integrin, αvβ3 was a surprise.[6]
It showed the molecule to be folded into an inverted V-shape which brings the ligand-binding sites close to the cell membrane. Perhaps more importantly, the crystal structure was also obtained for the same integrin bound to a small ligand containing the RGD-sequence, the drug cilengitide.[7] As detailed above, this finally revealed why divalent cations (in the A-domains) are critical for RGD-ligand binding to integrins. The interaction with such sequences is believed to be a primary switch by which ECM exerts its effects on cell behaviour.
The structure poses many questions, especially regarding ligand binding and signal transduction. The ligand binding site is directed towards the C-terminal of the integrin, the region where the molecule emerges from the cell membrane. If it emerges orthogonally from the membrane, the ligand binding site would apparently be obstructed, especially as integrin ligands are typically massive, and well cross-linked components of the ECM. In fact, little is known about the angle which membrane proteins subtend to the plane of the membrane—it is a problem difficult to address with available technologies. The default assumption is that they emerge rather like little lollipops—the evidence for this sweet supposition is noticeable by its absence. The integrin structure has drawn attention to this problem, which may have implications for how membrane proteins work.
Although the crystal structure changed surprisingly little after binding to cilengitide, the current hypothesis is that integrin function involves changes in shape to move the ligand binding site into a more accessible position away from the cell surface, and this shape change also triggers intracellular signaling. And there is a wide body of cell biological and biochemical literature that supports this view. Perhaps the most convincing evidence involves the use of antibodies that only recognize integrins when they have bound to their ligands, or are activated. As the "footprint" that an antibody makes on its binding target is roughly a circle about 3 nm in diameter, the resolution of this technique is low. Nevertheless, these so-called LIBS (Ligand-Induced-Binding-Sites) antibodies unequivocally show that dramatic changes in integrin shape routinely occur.
Function
Integrins have two main functions:
- Attachment of the cell to the ECM
- Signal transduction from the ECM to the cell
However, they are also involved in a wide range of other biological activities, including immune patrolling, cell migration, and binding to cells by certain viruses, such as adenovirus, echovirus, hantavirus, and foot and mouth disease viruses.
A prominent function of the integrins is seen in the molecule GPIIbIIIa, an integrin on the surface of blood platelets (thrombocytes) responsible for attachment to fibrin within a developing blood clot. This molecule dramatically increases its binding affinity for fibrin/fibrinogen through association of platelets with exposed collagen in the wound site. Upon association with the collagen, GPIIbIIIa undergoes a conformational change in its structure, allowing it to bind with fibrin and other blood components to form the clot matrix and stop blood loss.
Attachment of cell to the ECM
Integrins couple the ECM outside a cell to the cytoskeleton (in particular the microfilaments) inside the cell. Which ligand in the ECM the integrin can bind to is mainly decided by which α and β subunits the integrin is made of. Among the ligands of integrins are fibronectin, vitronectin, collagen, and laminin. The connection between the cell and the ECM may help the cell to endure pulling forces without being ripped out of the ECM. The ability of a cell to create this kind of bond is also of vital importance in ontogeny.
Cell attachment to the ECM is a basic requirement to build a multicellular organism. Integrins are not simply hooks, but give the cell critical signals about the nature of its surroundings. Together with signals arising from receptors for soluble growth factors like VEGF, EGF, and many others, they enforce a cellular decision on what biological action to take, be it attachment, movement, death, or differentiation. Thus integrins lie at the heart of many cellular biological processes. The attachment of the cell takes place through formation of cell adhesion complexes, which consist of integrins and many cytoplasmic proteins such as talin, vinculin, paxillin, and alpha-actinin. These act by regulating kinases such as FAK (focal adhesion kinase) and Src kinase family members to phosphorylate substrates such as p130CAS thereby recruiting signaling adaptors such as CRK. These adhesion complexes attach to the actin cytoskeleton. The integrins thus serve to link across the plasma membrane two networks: the extracellular ECM and the intracellular actin filamentous system.
One of the most important functions of surface integrins is their role in cell migration. Cells adhere to a substrate through their integrins. During movement, the cell makes new attachments to the substrate at its front and concurrently releases those at its rear. When released from the substrate, integrin molecules are taken back into the cell by endocytosis; they are transported through the cell to its front by the endocytic cycle where they are added back to the surface. In this way they are cycled for reuse, enabling the cell to make fresh attachments at its leading front.
Signal transduction
Integrins play an important role in cell signaling. Connection with ECM molecules can cause a signal to be relayed into the cell through protein kinases that are indirectly and temporarily connected with the intracellular end of the integrin molecule, likely following shape changes directly stimulated by ECM binding.
The signals the cell receives through the integrin can have relation to:
Vertebrate integrins
The following are some of the integrins found in vertebrates:
| Name | Synonyms | Distribution | Ligands |
| α1β1 | Many | Collagens, laminins.[8] | |
| α2β1 | Many | Collagens, laminins[8] | |
| α4β1 | VLA-4[8] | Hematopoietic cells | Fibronectin, VCAM-1[8] |
| α5β1 | fibronectin receptor | widespread | fibronectin[8] and proteinases |
| α6β1 | laminin receptor | widespread | matrix macromolecules laminins |
| αLβ2 | LFA-1[8] | T-lymphocytes | ICAM-1, ICAM-2[8] |
| αMβ2 | Mac-1, CR3[8] | Neutrophils and monocytes | Serum proteins, ICAM-1[8] |
| αIIbβ3 | Platelets[8] | fibrinogen, fibronectin[8] | |
| αVβ3 | vitronectin receptor[9] | activated endothelial cells, melanoma, glioblastoma | vitronectin,[9] fibronectin, fibrinogen, osteopontin, Cyr61 |
| αVβ5 | widespread, esp. fibroblasts, epithelial cells | vitronectin and adenovirus | |
| αVβ6 | proliferating epithelia, esp. lung and liver | fibronectin; TGFβ1+3 | |
| α6β4 | Epithelial cells[8] | Laminin[8] |
Beta1 integrins interact with many alpha integrin chains. Gene knockouts of integrins in mice are not always lethal. It proves that during embryonal development, one integrin may substitute its function for another, to allow survival. Some integrins are on the cell surface in an inactive state, and can be rapidly primed, or put into a state capable of binding their ligands, by cytokines. Integrins can assume several different well defined shapes, or "conformational states". Once primed, the conformational state changes to stimulate ligand binding which then activates the receptors, also by inducing a shape change, to trigger outside-in signal transduction.
Additional images
 Integrin (captions in German) |
References
- ↑ Sauer FG, Fütterer K, Pinkner JS, Dodson KW, Hultgren SJ, Waksman G (August 1999). "Structural basis of chaperone function and pilus biogenesis". Science 285 (5430): 1058–61. doi:10.1126/science.285.5430.1058. PMID 10446050.
- ↑ Xiong JP, Stehle T, Diefenbach B, et al. (October 2001). "Crystal structure of the extracellular segment of integrin alpha Vbeta3". Science 294 (5541): 339–45. doi:10.1126/science.1064535. PMID 11546839.
- ↑ Humphries M.J. (2000). "Integrin structure". Biochem. Soc. Trans. 28 (4): 311–339. doi:10.1042/0300-5127:0280311. PMID 10961914.
- ↑ Nermut MV, Green NM, Eason P, Yamada SS, Yamada KM (December 1988). "Electron microscopy and structural model of human fibronectin receptor". EMBO J. 7 (13): 4093–9. PMID 2977331.
- ↑ Hynes R (2002). "Integrins: bidirectional, allosteric signaling machines". Cell 110 (6): 673–87. doi:10.1016/S0092-8674(02)00971-6. PMID 12297042.
- ↑ Xiong JP; Stehle, T; Diefenbach, B; Zhang, R; Dunker, R; Scott, DL; Joachimiak, A; Goodman, SL et al. (2001). "Crystal structure of the extracellular segment of integrin αvβ3". Science 294 (5541): 339–345. doi:10.1126/science.1064535. PMID 11546839.
- ↑ Smith J (2003). "Cilengitide Merck". Curr Opin Investig Drugs 4 (6): 741–5. PMID 12901235.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 Molecular cell biology. Lodish, Harvey F. 5. ed. : – New York : W. H. Freeman and Co., 2003, 973 s. b ill. ISBN 0-7167-4366-3
- ↑ 9.0 9.1 Hermann P, Armant M, Brown E, Rubio M, Ishihara H, Ulrich D, Caspary RG, Lindberg FP, Armitage R, Maliszewski C, Delespesse G, Sarfati M (February 1999). "The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23". J. Cell Biol. 144 (4): 767–75. doi:10.1083/jcb.144.4.767. PMID 10037797. PMC 2132927. http://www.jcb.org/cgi/pmidlookup?view=long&pmid=10037797.
External links
 Media related to Integrins at Wikimedia Commons
Media related to Integrins at Wikimedia Commons- The Integrin Protein
- Talin substrate for calpain – PMAP The Proteolysis Map animation.
- MeSH Integrins
|
||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||