Indole
| Indole | |
|---|---|
 |
|
|
Indole
|
|
|
Other names
2,3-Benzopyrrole, ketole,
1-benzazole |
|
| Identifiers | |
| CAS number | 120-72-9 |
| ChemSpider | 776 |
| RTECS number | NL2450000 |
|
SMILES
C1(NC=C2)=C2C=CC=C1
|
|
| Properties | |
| Molecular formula | C8H7N |
| Molar mass | 117.15 g/mol |
| Appearance | White solid |
| Density | 1.1747 g/cm3, solid |
| Melting point |
52 - 54°C (326 K) |
| Boiling point |
253 - 254°C (526 K) |
| Solubility in water | 0.19 g/100 ml (20 °C) Soluble in hot water |
| Acidity (pKa) | 16.2 (21.0 in DMSO) |
| Basicity (pKb) | 17.6 |
| Structure | |
| Crystal structure | ? |
| Molecular shape | Planar |
| Dipole moment | 2.11 D in benzene |
| Hazards | |
| MSDS | [1] |
| R/S statement | R: 21/22-37/38-41-50/53 S: 26-36/37/39-60-61 |
| Flash point | 121°C |
| Related compounds | |
| Related aromatic compounds |
benzene, benzofuran, carbazole, carboline, indene, indoline, isatin, methylindole, oxindole, pyrrole, skatole |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an indole ring are called indoles. The most famous derivative is the amino acid tryptophan, the precursor of neurotransmitter serotonin. Recently, indole has been validated as a privileged structure, a scaffold capable of providing useful ligands for diverse receptors. [1]
Contents |
General properties and occurrence
Indole is a solid at room temperature. Indole can be produced by bacteria as a degradation product of the amino acid tryptophan. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell[2], and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar.
Indole undergoes electrophilic substitution, mainly at position 3. Substituted indoles are structural elements of (and for some compounds the synthetic precursors for) the tryptophan-derived tryptamine alkaloids like the neurotransmitter serotonin, and melatonin. Other indolic compounds include the plant hormone Auxin (indolyl-3-acetic acid, IAA), the anti-inflammatory drug indomethacin, and the betablocker pindolol.
The name indole is a portmanteau of the words indigo and oleum, since indole was first isolated by treatment of the indigo dye with oleum.
History

Indole chemistry began to develop with the study of the dye indigo. Indigo can be converted to isatin and then to oxindole. Then, in 1866, Adolf von Baeyer reduced oxindole to indole using zinc dust.[3] In 1869, he proposed a formula for indole (left).[4]
Certain indole derivatives were important dyestuffs until the end of the 19th century. In the 1930s, interest in indole intensified when it became known that the indole nucleus is present in many important alkaloids, as well is in tryptophan and auxins, and it remains an active area of research today.[5]
Synthesis of indoles
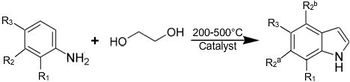
Indole is a major constituent of coal-tar, and the 220-260 °C distillation fraction is the main industrial source of the material. Indole and its derivatives can also be synthesized by a variety of methods.[6][7][8] The main industrial routes start from aniline.
Illustrative of such large-scale syntheses, indole (and substituted derivatives) form via vapor-phase reaction of aniline with ethylene glycol in the presence of catalysts:
Reactions are generally conducted between 200 and 500 °C. Yields can be as high as 60%. Other precursors to indole include formyltoluidine, 2-ethylaniline, and 2-(2-nitrophenyl)ethanol, all of which undergo cylizations.[9] Many other methods have been developed that are applicable.
Leimgruber-Batcho indole synthesis
The Leimgruber-Batcho indole synthesis is an efficient method of sythesizing indole and substituted indoles. Originally disclosed in a patent in 1976, this method is high-yielding and can generate substituted indoles. This method is especially popular in the pharmaceutical industry, where many pharmaceutical drugs are made up of specifically substituted indoles.
Fischer indole synthesis
One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis developed in 1883 by Emil Fischer. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions. Indole can still be synthesized however using the Fischer indole synthesis by reacting phenylhydrazine with pyruvic acid followed by decarboxylation of the formed indole-2-carboxylic acid. This has also been accomplished in a one-pot synthesis using microwave irradiation.[10]
Other indole forming reactions
- Bartoli indole synthesis
- Bischler-Möhlau indole synthesis
- Fukuyama indole synthesis
- Gassman indole synthesis
- Hemetsberger indole synthesis
- Larock indole synthesis
- Madelung synthesis
- Nenitzescu indole synthesis
- Reissert indole synthesis
- Baeyer-Emmerling indole synthesis
- In the Diels-Reese reaction[11][12] dimethyl acetylenedicarboxylate reacts with diphenylhydrazine to an adduct which in xylene gives dimethyl indole-2,3-dicarboxylate and aniline. With other solvents other products are formed: with glacial acetic acid a pyrazolone and with pyridine a quinoline.
Chemical reactions of indole
Basicity
Unlike most amines, indole is not basic. The bonding situation is completely analogous to that in pyrrole. Very strong acids such as hydrochloric acid are required to protonate indole. The protonated form has an pKa of -3.6. The sensitivity of many indolic compounds (e.g., tryptamines) under acidic conditions is caused by this protonation.
Electrophilic substitution
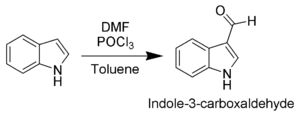
The most reactive position on indole for electrophilic aromatic substitution is C-3, which is 1013 times more reactive than benzene . For example, Vilsmeier-Haack formylation of indole[13] will take place at room temperature exclusively at C-3. Since the pyrrollic ring is the most reactive portion of indole, nucleophilic substitution of the carbocyclic (benzene) ring can take place only after N-1, C-2, and C-3 are substituted.
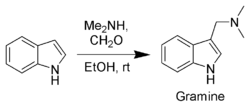
Gramine, a useful synthetic intermediate, is produced via a Mannich reaction of indole with dimethylamine and formaldehyde. It is the precursor to indole acetic acid and synthetic tryptophan.
Nitrogen-H acidity and organometallic indole anion complexes
The N-H center has a pKa of 21 in DMSO, so that very strong bases such as sodium hydride or butyl lithium and water-free conditions are required for complete deprotonation. The resulting alkali metal derivatives can react in two ways. The more ionic salts such as the sodium or potassium compounds tend to react with electrophiles at nitrogen-1, whereas the more covalent magnesium compounds (indole Grignard reagents) and (especially) zinc complexes tend to react at carbon-3 (see figure below). Analogously, polar aprotic solvents such as DMF and DMSO tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene favour C-3 attack.[14]
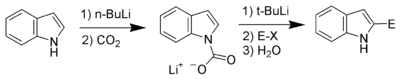
Carbon acidity and C-2 lithiation
After the N-H proton, the hydrogen at C-2 is the next most acidic proton on indole. Reaction of N-protected indoles with butyl lithium or lithium diisopropylamide results in lithiation exclusively at the C-2 position. This strong nucleophile can then be used as such with other electrophiles.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole.[15]
Alan Katritzky also developed a technique for lithiating the 2-position of unsubstituted indole.[16]
Oxidation of indole
Due to the electron-rich nature of indole, it is easily oxidized. Simple oxidants such as N-bromosuccinimide will selectively oxidize indole 1 to oxindole (4 and 5).
Cycloadditions of indole
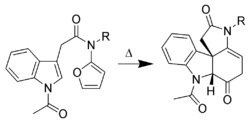
Only the C-2 to C-3 pi-bond of indole is capable of cycloaddition reactions. Intermolecular cycloadditions are not favorable, whereas intramolecular variants are often high-yielding. For example, Padwa et al.[17] have developed this Diels-Alder reaction to form advanced strychnine intermediates. In this case, the 2-aminofuran is the diene, whereas the indole is the dienophile.
Indoles also undergo intramolecular [2+3] and [2+2] cycloadditions.
Applications
Natural jasmine oil, used in the perfume industry, contains around 2.5% of indole. Since 1 kilogram of the natural oil requires processing several million jasmine blossoms and costs around $10,000, indole (among other things) is used in the manufacture of synthetic jasmine oil (which costs around $10/kg).
See also
- Martinet dioxindole synthesis
- Skatole (3-methylindole)
- Stollé synthesis
- Tryptamines
General references
- Indoles Part One, W. J. Houlihan (ed.), Wiley Interscience, New York, 1972.
- Sundberg, R. J. (1996). Indoles. San Diego: Academic Press. ISBN 0-12-676945-1.
- Joule, J. A.; Mills, K. (2000). Heterocyclic Chemistry. Oxford, UK: Blackwell Science. ISBN 0-632-05453-0.
- Joule, J., In Science of Synthesis, Thomas, E. J., Ed.; Thieme: Stuttgart, (2000); Vol. 10, p. 361. ISBN 3-13-112241-2 (GTV); ISBN 0-86577-949-X (TNY).
References
- ↑ Fernando R. De Sá Alves, Carlos A. M. Fraga, and Eliezer J. Barreiro (2009). "From Nature to Drug Discovery: The Indole Scaffold as a Privileged Structure". Mini Rev. Med. Chem. 9: 782–793. doi:10.2174/138955709788452649.
- ↑ http://www.leffingwell.com/olfact5.htm
- ↑ Baeyer, A. (1866). "Ueber die Reduction aromatischer Verbindungen mittelst Zinkstaub". Ann. 140: 295. doi:10.1002/jlac.18661400306.
- ↑ Baeyer, A.; Emmerling, A. (1869). "Synthese des Indols". Chemische Berichte 2: 679. doi:10.1002/cber.186900201268.
- ↑ R. B. Van Order, H. G. Lindwall (1942). "Indole". Chem. Rev. 30: 69–96. doi:10.1021/cr60095a004.
- ↑ Gribble G. W. (2000). "Recent developments in indole ring synthesis—methodology and applications". J. Chem. Soc. Perkin Trans. 1: 1045. doi:10.1039/a909834h.
- ↑ Cacchi, S.; Fabrizi, G. (2005). "Synthesis and Functionalization of Indoles Through Palladium-catalyzed Reactions". Chem. Rev. 105: 2873. doi:10.1021/cr040639b.
- ↑ Humphrey, G. R.; Kuethe, J. T. (2006). "Practical Methodologies for the Synthesis of Indoles". Chem. Rev. 106: 2875. doi:10.1021/cr0505270.
- ↑ Gerd Collin and Hartmut Höke “Indole” Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_167.
- ↑ http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6THS-4R9JTR2-7&_user=10&_coverDate=02%2F04%2F2008&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=1180603862&_rerunOrigin=google&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=e045c5da1c003eaef1639e1945c57b77,A new and efficient one-pot synthesis of indoles,George Bratulescu,University of Craiova, Faculty of Chemistry,8 December 2007
- ↑ Diels, Otto; Reese, Johannes (1934). "Synthesen in der hydroaromatischen Reihe. XX. Über die Anlagerung von Acetylen-dicarbonsäureester an Hydrazobenzol". Ann. 511: 168. doi:10.1002/jlac.19345110114.
- ↑ Ernest H. Huntress, Joseph Bornstein, and William M. Hearon (1956). "An Extension of the Diels-Reese Reaction". J. Am. Chem. Soc. 78: 2225. doi:10.1021/ja01591a055.
- ↑ James, P. N.; Snyder, H. R. (1959). "Indole-3-aldehyde". Organic Syntheses 39: 30. http://www.orgsyn.org/orgsyn/prep.asp?prep=cv4p0539.
- ↑ Heaney, H.; Ley, S. V. (1974). "1-Benzylindole". Organic Syntheses 54: 58. http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p0104.
- ↑ Bergman, J.; Venemalm, L. (1992). "Efficient synthesis of 2-chloro-, 2-bromo-, and 2-iodoindole". J. Org. Chem. 57: 2495. doi:10.1021/jo00034a058.
- ↑ Alan R. Katritzky, Jianqing Li, Christian V. Stevens (1995). "Facile Synthesis of 2-Substituted Indoles and Indolo[3,2-bcarbazoles from 2-(Benzotriazol-1-ylmethyl)indole"]. J. Org. Chem. 60 (11): 3401–3404. doi:10.1021/jo00116a026. http://pubs.acs.org/doi/abs/10.1021/jo00116a026.
- ↑ Lynch, S. M. ; Bur, S. K.; Padwa, A. (2002). "Intramolecular Amidofuran Cycloadditions across an Indole π-Bond: An Efficient Approach to the Aspidosperma and Strychnos ABCE Core". Org. Lett. 4: 4643. doi:10.1021/ol027024q.
See also
- Indole-3-butyric acid
- Indole test - biochemical test for bacterial identification