Growth hormone
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Growth hormone (GH) is a protein-based peptide hormone. It stimulates growth, cell reproduction and regeneration in humans and other animals. Growth hormone is a 191-amino acid, single-chain polypeptide which is synthesized, stored, and secreted by the somatotroph cells within the lateral wings of the anterior pituitary gland. Somatotropin refers to the growth hormone produced natively and naturally in animals, whereas the term somatropin refers to growth hormone produced by recombinant DNA technology,[1] and is abbreviated "HGH" in humans.
Growth hormone is used in medicine to treat children's growth disorders and adult growth hormone deficiency. In recent years, growth hormone replacement therapies have become popular in the battle against aging and obesity. Reported effects on GH-deficient patients (but not on healthy people) include decreased body fat, increased muscle mass, increased bone density, increased energy levels, improved skin tone and texture, increased sexual function and improved immune system function. At this time, hGH is still considered a very complex hormone, and many of its functions are still unknown.[2]
In its role as an anabolic agent, HGH has been used by competitors in sports since the 1970s, and it has been banned by the IOC and NCAA. Traditional urine analysis could not detect doping with hGH, so the ban was unenforceable until the early 2000s when blood tests that could distinguish between natural and artificial hGH were starting to be developed. Blood tests conducted by WADA at the 2004 Olympic Games in Athens, Greece primarily targeted hGH.[2]
Contents |
Gene locus
Genes for human growth hormone, known as growth hormone 1 (somatotropin) and growth hormone 2, are localized in the q22-24 region of chromosome 17 and are closely related to human chorionic somatomammotropin (also known as placental lactogen) genes. GH, human chorionic somatomammotropin, and prolactin (PRL) are a group of homologous hormones with growth-promoting and lactogenic activity.
Structure
The major isoform of the human growth hormone is a protein of 191 amino acids and a molecular weight of 22,124 daltons. The structure includes four helices necessary for functional interaction with the GH receptor. It appears that, in structure, GH is evolutionarily homologous to prolactin and chorionic somatomammotropin. Despite marked structural similarities between growth hormone from different species, only human and primate growth hormones have significant effects in humans.
Several molecular isoforms of GH circulate in the plasma. A percentage of the growth hormone in the circulation is bound to a protein (growth hormone binding protein, GHBP) which is the truncated part of the growth hormone receptor, and an acid labile subunit (ALS).
Regulation
Peptides released by neurosecretory nuclei of the hypothalamus (Growth hormone-releasing hormone/somatocrinin and Growth hormone-inhibiting hormone/somatostatin) into the hypophyseal portal venous blood surrounding the pituitary are the major controllers of GH secretion by the somatotropes. However, although the balance of these stimulating and inhibiting peptides determines GH release, this balance is affected by many physiological stimulators (e.g., exercise, nutrition, sleep) and inhibitors of GH secretion (e.g., Free fatty acids)[3] Stimulators of HGH secretion include:
- peptide hormones
- sex hormones[6]
- clonidine and L-DOPA by stimulating GHRH release[7]
- hypoglycemia, arginine[8] and propranolol by inhibiting somatostatin release[7]
Inhibitors of GH secretion include:
- somatostatin from the periventricular nucleus [12]
- circulating concentrations of GH and IGF-1 (negative feedback on the pituitary and hypothalamus)[2]
- hyperglycemia[7]
- glucocorticoids[13]
- dihydrotestosterone
In addition to control by endogenous and stimulus processes, a number of foreign compounds (xenobiotics such as drugs and endocrine disruptors) are known to influence GH secretion and function.[14]
Secretion patterns
HGH is synthesized and secreted from the anterior pituitary gland in a pulsatile manner throughout the day; surges of secretion occur at 3- to 5-hour intervals.[2] The plasma concentration of GH during these peaks may range from 5 to even 45 ng/mL.[15] The largest and most predictable of these GH peaks occurs about an hour after onset of sleep.[16] Otherwise there is wide variation between days and individuals. Nearly fifty percent of HGH secretion occurs during the third and fourth REM sleep stages.[17] Between the peaks, basal GH levels are low, usually less than 5 ng/mL for most of the day and night.[16] Additional analysis of the pulsatile profile of GH described in all cases less than 1 ng/ml for basal levels while maximum peaks were situated around 10-20 ng/mL.[18][19]
A number of factors are known to affect HGH secretion, such as age, gender, diet, exercise, stress, and other hormones.[2] Young adolescents secrete HGH at the rate of about 700 μg/day, while healthy adults secrete HGH at the rate of about 400 μg/day.[20]
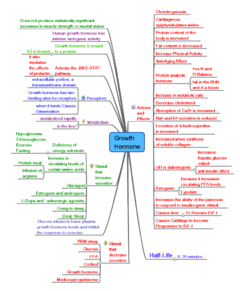
Functions of GH

Effects of growth hormone on the tissues of the body can generally be described as anabolic (building up). Like most other protein hormones, GH acts by interacting with a specific receptor on the surface of cells.
Increased height during childhood is the most widely known effect of GH. Height appears to be stimulated by at least two mechanisms:
- Because polypeptide hormones are not fat-soluble, they cannot penetrate sarcolemma. Thus, GH exerts some of its effects by binding to receptors on target cells, where it activates the MAPK/ERK pathway.[21] Through this mechanism GH directly stimulates division and multiplication of chondrocytes of cartilage.
- GH also stimulates, through the JAK-STAT signaling pathway,[21] the production of insulin-like growth factor 1 (IGF-1, formerly known as somatomedin C), a hormone homologous to proinsulin.[22] The liver is a major target organ of GH for this process and is the principal site of IGF-1 production. IGF-1 has growth-stimulating effects on a wide variety of tissues. Additional IGF-1 is generated within target tissues, making it what appears to be both an endocrine and an autocrine/paracrine hormone. IGF-1 also has stimulatory effects on osteoblast and chondrocyte activity to promote bone growth.
In addition to increasing height in children and adolescents, growth hormone has many other effects on the body:
- Increases calcium retention, and strengthens and increases the mineralization of bone
- Increases muscle mass through sarcomere hyperplasia
- Promotes lipolysis
- Increases protein synthesis
- Stimulates the growth of all internal organs excluding the brain
- Plays a role in fuel homeostasis
- Reduces liver uptake of glucose
- Promotes gluconeogenesis in the liver[23]
- Contributes to the maintenance and function of pancreatic islets
- Stimulates the immune system
Excesses
The most common disease of GH excess is a pituitary tumor composed of somatotroph cells of the anterior pituitary. These somatotroph adenomas are benign and grow slowly, gradually producing more and more GH. For years, the principal clinical problems are those of GH excess. Eventually the adenoma may become large enough to cause headaches, impair vision by pressure on the optic nerves, or cause deficiency of other pituitary hormones by displacement.
Prolonged GH excess thickens the bones of the jaw, fingers and toes. Resulting heaviness of the jaw and increased size of digits is referred to as acromegaly. Accompanying problems can include sweating, pressure on nerves (e.g., carpal tunnel syndrome), muscle weakness, excess sex hormone binding globulin (SHBG), insulin resistance or even a rare form of type 2 diabetes, and reduced sexual function.
GH-secreting tumors are typically recognized in the fifth decade of life. It is extremely rare for such a tumor to occur in childhood, but, when it does, the excessive GH can cause excessive growth, traditionally referred to as pituitary gigantism.
Surgical removal is the usual treatment for GH-producing tumors. In some circumstances, focused radiation or a GH antagonist such as pegvisomant may be employed to shrink the tumor or block function. Other drugs like octreotide (somatostatin agonist) and bromocriptine (dopamine agonist) can be used to block GH secretion because both somatostatin and dopamine negatively inhibit GHRH-mediated GH release from the anterior pituitary.
Deficiencies
The effects of growth hormone deficiency vary depending on the age at which they occur. In children, growth failure and short stature are the major manifestations of GH deficiency, with common causes including genetic conditions and congenital malformations. It can also cause delayed sexual maturity. In adults, deficiency is rare,[24] with the most common cause a pituitary adenoma, and others including a continuation of a childhood problem, other structural lesions or trauma, and very rarely idiopathic GHD.
Adults with GHD present with non-specific problems including truncal obesity with a relative decrease in muscle mass and, in many instances, decreased energy and quality of life.[24]
Diagnosis of GH deficiency involves a multiple-step diagnostic process, usually culminating in GH stimulation tests to see if the patient's pituitary gland will release a pulse of GH when provoked by various stimuli.
Treatment with exogenous GH is indicated only in limited circumstances,[24] and needs regular monitoring due to the frequency and severity of side-effects. GH is used as replacement therapy in adults with GH deficiency of either childhood-onset (after completing growth phase) or adult-onset (usually as a result of an acquired pituitary tumor). In these patients, benefits have variably included reduced fat mass, increased lean mass, increased bone density, improved lipid profile, reduced cardiovascular risk factors, and improved psychosocial well-being.
Therapeutic use
GH can be used to treat conditions that produce short stature but are not related to deficiencies in GH. However, results are not as dramatic when compared to short stature that is solely attributable to deficiency of GH. Examples of other causes of shortness often treated with GH are Turner syndrome, chronic renal failure, Prader–Willi syndrome, intrauterine growth retardation, and severe idiopathic short stature. Higher ("pharmacologic") doses are required to produce significant acceleration of growth in these conditions, producing blood levels well above normal ("physiologic"). Despite the higher doses, side-effects during treatment are rare, and vary little according to the condition being treated.
GH treatment improves muscle strength and slightly reduces body fat in Prader-Willi syndrome, which are significant concerns beyond the need to increase height. GH is also useful in maintaining muscle mass in wasting due to AIDS. GH can also be used in patients with short bowel syndrome to lessen the requirement for intravenous total parenteral nutrition.
GH can also be used for conditions that do not cause short stature. Typically, growth hormone treatment for conditions unrelated to stature is controversial and experimental. GH has been used for remission of multiple sclerosis, to reverse the effects of aging in older adults (see below), to enhance weight loss in obesity, as well as fibromyalgia, heart failure, Crohn's disease and ulcerative colitis, burns and bodybuilding or athletic enhancement.
Anti-aging agent
Claims for GH as an anti-aging treatment date back to 1990 when the New England Journal of Medicine published a study wherein GH was used to treat 12 men over 60.[25] At the conclusion of the study, all the men showed statistically significant increases in lean body mass and bone mineral, while the control group did not. The authors of the study noted that these improvements were the opposite of the changes that would normally occur over a 10- to 20-year aging period. Despite the fact the authors at no time claimed that GH had reversed the aging process itself, their results were misinterpreted as indicating that GH is an effective anti-aging agent.[26][27][28] This has led to organizations such as the controversial American Academy of Anti-Aging Medicine promoting the use of this hormone as an "anti-aging agent".[29]
A Stanford University School of Medicine survey of clinical studies on the subject published in early 2007 showed that the application of GH on healthy elderly patients increased muscle by about 2 kg and decreased body fat by the same amount.[26] However, these were the only positive effects from taking GH. No other critical factors were affected, such as bone density, cholesterol levels, lipid measurements, maximal oxygen consumption, or any other factor that would indicate increased fitness.[26] Researchers also did not discover any gain in muscle strength, which led them to believe that GH merely let the body store more water in the muscles rather than increase muscle growth. This would explain the increase in lean body mass.
Athletic enhancement
Athletes in many sports use human growth hormone to enhance their athletic performance. Some recent studies have not been able to support claims that human growth hormone can improve the athletic performance of professional male athletes.[30][31]
Side-effects
There is theoretical concern that HGH treatment may increase the risks of diabetes, especially in those with other predispositions treated with higher doses. If used for training, growth at a young age (25 or less) can cause severe symptoms. One survey of adults that had been treated with replacement cadaver GH (which has not been used anywhere in the world since 1985) during childhood showed a mildly increased incidence of colon cancer and prostate cancer, but linkage with the GH treatment was not established.[32]
Regular application of extra GH may show several negative side-effects such as joint swelling, joint pain, carpal tunnel syndrome, and an increased risk of diabetes.[26] Other side effects can include less sleep needed after dosing. This is common initially and decreases in effect after habitual use of GH.
History
The identification, purification and later synthesis of growth hormone is associated with Choh Hao Li. Genentech pioneered the first use of recombinant human growth hormone for human therapy in 1981.
Prior to its production by recombinant DNA technology, growth hormone used to treat deficiencies was extracted from the pituitary glands of cadavers. Attempts to create a wholly synthetic HGH failed. Limited supplies of HGH resulted in the restriction of HGH therapy to the treatment of idiopathic short stature.[33] Furthermore, growth hormone from other primates was found to be inactive in humans.[34]
In 1985, unusual cases of Creutzfeldt-Jacob disease were found in individuals that had received cadaver-derived HGH ten to fifteen years previously. Based on the assumption that infectious prions causing the disease were transferred along with the cadaver-derived HGH, cadaver-derived HGH was removed from the market.[20]
In 1985, biosynthetic human growth hormone replaced pituitary-derived human growth hormone for therapeutic use in the U.S. and elsewhere.
As of 2005, recombinant growth hormones available in the United States (and their manufacturers) included Nutropin (Genentech), Humatrope (Lilly), Genotropin (Pfizer), Norditropin (Novo), and Saizen (Merck Serono). In 2006, the U.S. Food and Drug Association (FDA) approved a version of rhGH called Omnitrope (Sandoz). A sustained-release form of growth hormone, Nutropin Depot (Genentech and Alkermes) was approved by the FDA in 1999, allowing for fewer injections (every 2 or 4 weeks instead of daily); however, the product was discontinued in 2004.
References
- ↑ Daniels ME (1992). "Lilly's Humatrope Experience". Nature Biotechnology 10: 812. doi:10.1038/nbt0792-812a.
- ↑ 2.0 2.1 2.2 2.3 2.4 Powers M (2005). "Performance-Enhancing Drugs". In Deidre Leaver-Dunn; Joel Houglum; Harrelson, Gary L.. Principles of Pharmacology for Athletic Trainers. Slack Incorporated. pp. 331–332. ISBN 1-55642-594-5.
- ↑ Bartholomew, Edwin F.; Martini, Frederic; Judi Lindsley Nath (2009). Fundamentals of anatomy & physiology. Upper Saddle River, NJ: Pearson Education Inc. pp. 616-617. ISBN 0-321-53910-9.
- ↑ Lin-Su K, Wajnrajch MP (December 2002). "Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor". Rev Endocr Metab Disord 3 (4): 313–23. doi:10.1023/A:1020949507265. PMID 12424433.
- ↑ Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR (November 2000). "The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion". Endocrinology 141 (11): 4325–8. doi:10.1210/en.141.11.4325. PMID 11089570.
- ↑ Meinhardt UJ, Ho KK (October 2006). "Modulation of growth hormone action by sex steroids". Clin. Endocrinol. (Oxf) 65 (4): 413–22. doi:10.1111/j.1365-2265.2006.02676.x. PMID 16984231.
- ↑ 7.0 7.1 7.2 Low LC (1991). "Growth hormone-releasing hormone: clinical studies and therapeutic aspects". Neuroendocrinology 53 Suppl 1: 37–40. PMID 1901390.
- ↑ Alba-Roth J, Müller OA, Schopohl J, von Werder K (December 1988). "Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion". J. Clin. Endocrinol. Metab. 67 (6): 1186–9. doi:10.1126/science.2237411. PMID 2903866.
- ↑ Van Cauter E, Latta F, Nedeltcheva A, Spiegel K, Leproult R, Vandenbril C, Weiss R, Mockel J, Legros JJ, Copinschi G (June 2004). "Reciprocal interactions between the GH axis and sleep". Growth Horm. IGF Res. 14 Suppl A: S10–7. doi:10.1016/j.ghir.2004.03.006. PMID 15135771.
- ↑ Nørrelund H (April 2005). "The metabolic role of growth hormone in humans with particular reference to fasting". Growth Horm. IGF Res. 15 (2): 95–122. doi:10.1016/j.ghir.2005.02.005. PMID 15809014.
- ↑ Kanaley JA, Weltman JY, Veldhuis JD, Rogol AD, Hartman ML, Weltman A (November 1997). "Human growth hormone response to repeated bouts of aerobic exercise". J. Appl. Physiol. 83 (5): 1756–61. PMID 9375348. http://jap.physiology.org/cgi/content/full/83/5/1756.
- ↑ Guillemin R, Gerich JE (1976). "Somatostatin: physiological and clinical significance". Annu. Rev. Med. 27: 379–88. doi:10.1146/annurev.me.27.020176.002115. PMID 779605.
- ↑ Allen DB (September 1996). "Growth suppression by glucocorticoid therapy". Endocrinol. Metab. Clin. North Am. 25 (3): 699–717. doi:10.1016/S0889-8529(05)70348-0. PMID 8879994.
- ↑ Scarth JP (2006). "Modulation of the growth hormone-insulin-like growth factor (GH-IGF) axis by pharmaceutical, nutraceutical and environmental xenobiotics: an emerging role for xenobiotic-metabolizing enzymes and the transcription factors regulating their expression. A review". Xenobiotica 36 (2-3): 119–218. doi:10.1080/00498250600621627. PMID 16702112.
- ↑ Natelson BH, Holaday J, Meyerhoff J, Stokes PE (August 1975). "Temporal changes in growth hormone, cortisol, and glucose: relation to light onset and behavior". Am. J. Physiol. 229 (2): 409–15. PMID 808970. http://ajplegacy.physiology.org/cgi/content/abstract/229/2/409.
- ↑ 16.0 16.1 Takahashi Y, Kipnis D, Daughaday W (1968). "Growth hormone secretion during sleep". J Clin Invest 47 (9): 2079–90. doi:10.1172/JCI105893. PMID 5675428.
- ↑ Mehta, Ameeta and Hindmarsh, Peter. 2002. The use of somatropin (recombinant growth hormone) in children of short stature. Pediatric Drugs. 4: 37-47.
- ↑ Nindl BC, Hymer WC, Deaver DR, Kraemer WJ (1 July 2001). "Growth hormone pulsatility profile characteristics following acute heavy resistance exercise". J. Appl. Physiol. 91 (1): 163–72. PMID 11408427. http://jap.physiology.org/cgi/content/abstract/91/1/163.
- ↑ Juul A, Jørgensen JO, Christiansen JS, Müller J, Skakkeboek NE (1995). "Metabolic effects of GH: a rationale for continued GH treatment of GH-deficient adults after cessation of linear growth". Horm. Res. 44 Suppl 3: 64–72. doi:10.1159/000184676. PMID 8719443.
- ↑ 20.0 20.1 Gardner, David G., Shoback, Dolores (2007). Greenspan's Basic and Clinical Endocrinology (8th ed.). New York: McGraw-Hill Medical. pp. 193–201. ISBN 0-07-144011-9.
- ↑ 21.0 21.1 Binder G, Wittekindt N, Ranke MB (February 2007). "Noonan Syndrome: Genetics and Responsiveness to Growth Hormone Therapy". Horm Res 67 (Supplement 1): 45-49. doi:10.1159/000097552. http://books.google.com/books?id=nQ9ilbixQEgC&pg=PA46&lpg=PA46&dq=gh+growth+hormone+ras&source=bl&ots=HBZF1PLc9B&sig=bPvMyw75xRbDFt0xy0lKNuIviyk&hl=en&ei=bH7hS9PbI4KKlwfU09GFAg&sa=X&oi=book_result&ct=result&resnum=26&ved=0CKABEOgBMBk#v=onepage&q=gh%20growth%20hormone%20ras&f=false.
- ↑ "Actions of Anterior Pituitary Hormones: Physiologic Actions of GH". Medical College of Georgia. 2007. http://www.lib.mcg.edu/edu/eshuphysio/program/section5/5ch2/s5ch2_19.htm. Retrieved 2008-01-16.
- ↑ King, MW (2006). "Structure and Function of Hormones: Growth Hormone". Indiana State University. http://web.indstate.edu/thcme/mwking/peptide-hormones.html#gh. Retrieved 2008-01-16.
- ↑ 24.0 24.1 24.2 Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML; Endocrine Society's Clinical Guidelines Subcommittee, Stephens PA (May 2006). "Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline". J. Clin. Endocrino. Metab. 91 (5): 1621–34. doi:10.1210/jc.2005-2227. PMID 16636129.
- ↑ Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE (July 1990). "Effects of human growth hormone in men over 60 years old". N. Engl. J. Med. 323 (1): 1–6. PMID 2355952.
- ↑ 26.0 26.1 26.2 26.3 Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR (January 2007). "Systematic review: the safety and efficacy of growth hormone in the healthy elderly". Ann. Intern. Med. 146 (2): 104–15. PMID 17227934.
- ↑ "No proof that growth hormone therapy makes you live longer, study finds". PhysOrg.com. 2007-01-16. http://www.physorg.com/news88140162.html. Retrieved 2009-03-16.
- ↑ Gordon, Mark L. M.D. Human Growth Hormone (HGH) as an Anti-Aging Agent
- ↑ Kuczynski, Alex (12 April, 1998). "Anti-Aging Potion or Poison?". New York Times. http://www.nytimes.com/1998/04/12/style/anti-aging-potion-or-poison.html.
- ↑ http://www.bloomberg.com/apps/news?pid=20601124&sid=awlswGxIiU5c&refer=home
- ↑ http://grg51.typepad.com/steroid_nation/2008/03/review-from-sta.html
- ↑ Swerdlow AJ, Higgins CD, Adlard P, Preece MA (July 2002). "Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959-85: a cohort study". Lancet 360 (9329): 273–7. doi:10.1016/S0140-6736(02)09519-3. PMID 12147369.
- ↑ Maybe, Nancy G (1984). "Direct expression of human growth in Escherichia coli with the lipoprotein promoter". In Arthur P. Bollon. Recombinant DNA products: insulin, interferon, and growth hormone. Boca Raton: CRC Press. ISBN 0-8493-5542-7.
- ↑ Hintz, Raymond L. (1984). "Biological actions in humans of recombinant DNA synthesized human growth hormone". In Arthur P. Bollon. Recombinant DNA products: insulin, interferon, and growth hormone. Boca Raton: CRC Press. ISBN 0-8493-5542-7.
External links
- Magic Foundation, Support for adults and children affected with growth hormone deficiency.
- Height increase, Human Growth Hormone Aid For Children With Idiopathic Short Stature.