Golgi apparatus
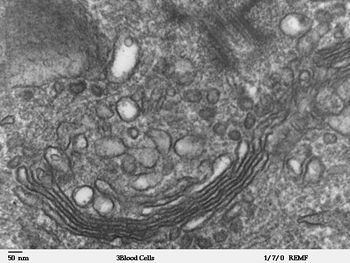
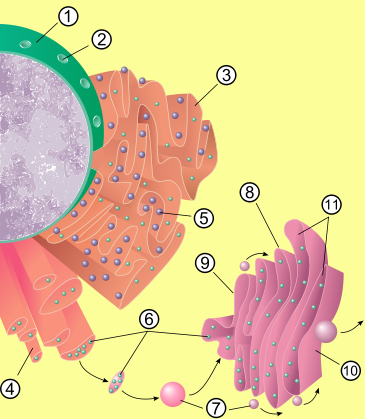
The Golgi apparatus (also Golgi body or Golgi Complex)[1] is an organelle found in most eukaryotic cells. It was identified in 1898 by the Italian physician Camillo Golgi and was named after him.
The primary function of the Golgi apparatus is to process and package macromolecules, such as proteins and lipids, after their synthesis and before they make their way to their destination; it is particularly important in the processing of proteins for secretion. The Golgi apparatus forms a part of the cellular endomembrane system.
Contents |
Discovery
Due to its fairly large size, the golgi apparatus was one of the first organelles to be discovered and observed in detail. The apparatus was discovered in 1897 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his microscope, he termed the structure the internal reticular apparatus. The structure was then renamed after Golgi not long after the announcement of his discovery in 1898. However, some doubted the discovery at first, arguing that the appearance of the structure was merely an optical illusion created by the observation technique used by Golgi. With the development of modern microscopes in the twentieth century, the discovery was confirmed.[2]
Structure
The Golgi is composed of stacks of membrane-bound structures known as cisternae (singular: cisterna). An individual stack is sometimes called a dictyosome (from Greek dictyon, net + soma, body)[3], especially in plant cells.[4] A mammalian cell typically contains 40 to 100 stacks.[5] Between four and eight cisternae are usually present in a stack; however, in some protists as many as sixty have been observed.[2] Each cisterna comprises a flattened membrane disk, and carries Golgi enzymes to help or to modify cargo proteins that travel through them. They are found in both plant and animal cells.
The cisternae stack has four functional regions: the cis-Golgi network, medial-Golgi, endo-Golgi, and trans-Golgi network. Vesicles from the endoplasmic reticulum (via the vesicular-tubular clusters) fuse with the network and subsequently progress through the stack to the trans Golgi network, where they are packaged and sent to the required destination. Each region contains different enzymes which selectively modify the contents depending on where they reside.[6] The cisternae also carry structural proteins important for their maintenance as flattened membranes which stack upon each other.
The trans face of the trans-Golgi network is the face from which vesicles leave the Golgi. These vesicles then proceed to later compartments such as the cell membrane, secretory vesicles or late endosomes.
New cisternae form at the cis-Golgi network. The cis- and trans-Golgi networks are thought to be specialised cisternae leading in and out of the Golgi apparatus.
Function
Cells synthesize a large number of different macromolecules. The Golgi apparatus is integral in modifying, sorting, and packaging these macromolecules for cell secretion (exocytosis) or use within the cell. It primarily modifies proteins delivered from the rough endoplasmic reticulum but is also involved in the transport of lipids around the cell, and the creation of lysosomes. In this respect it can be thought of as similar to a post office; it packages and labels items which it then sends to different parts of the cell.
Enzymes within the cisternae are able to modify substances by the addition of carbohydrates (glycosylation) and phosphates (phosphorylation). In order to do so, the Golgi imports substances such as nucleotide sugars from the cytosol. These modifications may also form a signal sequence which determines their final destination. For example, the Golgi apparatus adds a mannose-6-phosphate label to proteins destined for lysosomes.
The Golgi plays an important role in the synthesis of proteoglycans, which are molecules present in the extracellular matrix of animals. It is also a major site of carbohydrate synthesis.[7] This includes the productions of glycosaminoglycans (GAGs), long unbranched polysaccharides which the Golgi then attaches to a protein synthesised in the endoplasmic reticulum to form proteoglycans.[8] Enzymes in the Golgi polymerize several of these GAGs via a xylose link onto the core protein. Another task of the Golgi involves the sulfation of certain molecules passing through its lumen via sulphotranferases that gain their sulphur molecule from a donor called PAPs. This process occurs on the GAGs of proteoglycans as well as on the core protein. The level of sulfation is very important to the proteoglycans' signalling abilities as well as giving the proteoglycan its overall negative charge.[7]
The phosphorylation of molecules requires that ATP is imported into the lumen of the Golgi[9] and then utilised by resident kinases such as casein kinase 1 and casein kinase 2. One molecule that is phosphorylated in the Golgi is Apolipoprotein, which forms a molecule known as VLDL that is a constitute of blood serum. It is thought that the phosphorylation of these molecules is important to help aid in their sorting for secretion into the blood serum.[10]
The Golgi has a putative role in apoptosis, with several Bcl-2 family members localised there, as well as to the mitochondria. A newly characterized protein, GAAP (Golgi anti-apoptotic protein), almost exclusively resides in the Golgi and protects cells from apoptosis by an as-yet undefined mechanism (Gubser et al., 2007).
Vesicular transport
The vesicles that leave the rough endoplasmic reticulum are transported to the cis face of the Golgi apparatus, where they fuse with the Golgi membrane and empty their contents into the lumen. Once inside they are modified, sorted and shipped towards their final destination. As such, the Golgi apparatus tends to be more prominent and numerous in cells synthesising and secreting many substances: plasma B cells, the antibody-secreting cells of the immune system, have prominent Golgi complexes.
Those proteins destined for areas of the cell other than either the endoplasmic reticulum or Golgi apparatus are moved towards the trans face, to a complex network of membranes and associated vesicles known as the trans-Golgi network (TGN).[6] This area of the Golgi is the point at which proteins are sorted and shipped to their intended destinations by their placement into one of at least three different types of vesicles, depending upon the molecular marker they carry:[6]
| Type | Description | Example |
| Exocytotic vesicles (continuous) | Vesicle contains proteins destined for extracellular release. After packaging the vesicles bud off and immediately move towards the plasma membrane, where they fuse and release the contents into the extracellular space in a process known as constitutive secretion. | Antibody release by activated plasma B cells |
| Secretory vesicles (regulated) | Vesicle contains proteins destined for extracellular release. After packaging the vesicles bud off and are stored in the cell until a signal is given for their release. When the appropriate signal is received they move towards the membrane and fuse to release their contents. This process is known as regulated secretion. | Neurotransmitter release from neurons |
| Lysosomal vesicles | Vesicle contains proteins destined for the lysosome, an organelle of degradation containing many acid hydrolases, or to lysosome-like storage organelles. These proteins include both digestive enzymes and membrane proteins. The vesicle first fuses with the late endosome, and the contents are then transferred to the lysosome via unknown mechanisms. | Digestive proteases destined for the lysosome |
Transport mechanism
The transport mechanism which proteins use to progress through the Golgi apparatus is not yet clear; however a number of hypotheses currently exist. Until recently, the vesicular transport mechanism was favoured but now more evidence is coming to light to support cisternal maturation. The two proposed models may actually work in conjunction with each other, rather than being mutually exclusive. This is sometimes referred to as the combined model.[7]
- Cisternal maturation model: the cisternae of the Golgi apparatus move by being built at the cis face and destroyed at the trans face. Vesicles from the endoplasmic reticulum fuse with each other to form a cisterna at the cis face, consequently this cisterna would appear to move through the Golgi stack when a new cisterna is formed at the cis face. This model is supported by the fact that structures larger than the transport vesicles, such as collagen rods, were observed microscopically to progress through the Golgi apparatus.[7] This was initially a popular hypothesis, but lost favour in the 1980s. Recently it has made a comeback, as laboratories at the University of Chicago and the University of Tokyo have been able to use new technology to directly observe Golgi compartments maturing.[11] Additional evidence comes from the fact that COPI vesicles move in the retrograde direction, transporting Endoplasmic Reticulum proteins back to where they belong by recognizing a signal peptide.[12]
- Vesicular transport model: Vesicular transport views the Golgi as a very stable organelle, divided into compartments in the cis to trans direction. Membrane bound carriers transport material between the ER and the different compartments of the Golgi.[13] Experimental evidence includes the abundance of small vesicles (known technically as shuttle vesicles) in proximity to the Golgi apparatus. To direct the vesicles, actin filaments connect packaging proteins to the membrane to ensure that they fuse with the correct compartment.[7]
Golgi apparatus during mitosis
The Golgi apparatus will break up and disappear following the onset of mitosis, or cellular division. During the telophase of mitosis, the Golgi apparatus reappears; however, it is still uncertain how this occurs.[14]
Golgi apparatus in popular culture
"Golgi Apparatus" is the title of a song by the band Phish in their album Junta. Tom Marshall and guitarist Trey Anastasio wrote this with some friends during grade school and later reworked it as a Phish song.
External links
- Golgi antibody
- The Golgi Apparatus ASCB Image & Video Library
References
- ↑ [1]
- ↑ 2.0 2.1 "Molecular Expressions Cell Biology: The Golgi Apparatus". http://micro.magnet.fsu.edu/cells/golgi/golgiapparatus.html. Retrieved 2006-11-08.
- ↑ "Definition of dictyosome at dictionary.com". http://dictionary.reference.com/browse/dictyosome.
- ↑ Wolfe (1993). Molecular and Cellular Biology. Wadsworth. p. 828. ISBN 0-534-12408-9.
- ↑ Duran J, Kinseth M (2008 June). "The Role of GRASP55 in Golgi Fragmentation and Entry of Cells into Mitosis". Mol Biol Cell. 19 (6): 2579–2587. doi:10.1091/mbc.E07-10-0998. PMID 18385516. PMC 2397314. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2397314/.
- ↑ 6.0 6.1 6.2 Lodish; et al. (2004). Molecular Cell Biology (5th edn ed.). W.H. Freeman and Company. P0-7167-4366-3.
- ↑ 7.0 7.1 7.2 7.3 7.4 Alberts, Bruce; et al.. Molecular Biology of the Cell. Garland Publishing. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=cell.TOC.
- ↑ Prydz K, Dalen KT (15 January 2000). "Synthesis and sorting of proteoglycans". J. Cell. Sci. 113 Pt 2 (2): 193–205. PMID 10633071. http://jcs.biologists.org/cgi/pmidlookup?view=long&pmid=10633071.
- ↑ Capasso JM, Keenan TW, Abeijon C, Hirschberg CB (March 1989). "Mechanism of phosphorylation in the lumen of the Golgi apparatus. Translocation of adenosine 5'-triphosphate into Golgi vesicles from rat liver and mammary gland". J. Biol. Chem. 264 (9): 5233–40. PMID 2925690. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=2925690.
- ↑ Swift LL (December 1996). "Role of the Golgi apparatus in the phosphorylation of apolipoprotein B". J. Biol. Chem. 271 (49): 31491–5. doi:10.1074/jbc.271.49.31491 (inactive 2010-01-09). PMID 8940163. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=8940163.
- ↑ Glick, B.S. and Malhotra, V. (1998). "The curious status of the Golgi apparatus". Cell 95 (7): 883–889. doi:10.1016/S0092-8674(00)81713-4. PMID 9875843.
- ↑ Pelham, H.R.B. and J.E. Rothman, The Debate about Transport in the Golgi - Two Sides of the Same Coin? Cell, 2000. 102: p. 713-719.
- ↑ Glick, B.S., Organisation of the Golgi apparatus. Current Opinion in Cell Biology, 2000. 12: p. 450-456.
- ↑ http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/G/Golgi.html
|
||||||||||||||||||||||||||