gamma-Aminobutyric acid
| gamma-Aminobutyric acid | |
|---|---|
 |
|
 |
|
|
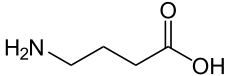
4-aminobutanoic acid
|
|
| Identifiers | |
| CAS number | 56-12-2 |
| PubChem | 119 |
| ChemSpider | 116 |
| MeSH | gamma-Aminobutyric+Acid |
| IUPHAR ligand | 1067 |
|
SMILES
C(CC(=O)O)CN
|
|
| Properties | |
| Molecular formula | C4H9NO2 |
| Molar mass | 103.12 g/mol |
| Melting point |
203.7 °C, 477 K, 399 °F |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
γ-Aminobutyric acid (GABA) (pronounced /ˈɡæmə əˈmiːnoʊbjuːˈtɪrɨk ˈæsɨd/, or the acronym pronounced /'gæbə/) is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system. In humans, GABA is also directly responsible for the regulation of muscle tone.[1] In insect species GABA acts only on excitatory nerve receptors.
Although chemically it is an amino acid, GABA is rarely referred to as such in the scientific or medical communities, because the term "amino acid," used without a qualifier, refers to the alpha amino acids, which GABA is not, nor is it incorporated into proteins.
In spastic diplegia in humans, GABA absorption becomes impaired by nerves damaged from the condition's upper motor neuron lesion, which leads to hypertonia of the muscles signaled by those nerves that can no longer absorb GABA.
Contents |
Function
Neurotransmitter
In vertebrates, GABA acts at inhibitory synapses in the brain by binding to specific transmembrane receptors in the plasma membrane of both pre- and postsynaptic neuronal processes. This binding causes the opening of ion channels to allow the flow of either negatively charged chloride ions into the cell or positively charged potassium ions out of the cell. Depending on which ion channels open, the membrane potential is either hyperpolarized or depolarized. This action results in a negative change in the transmembrane potential, usually causing hyperpolarization. Two general classes of GABA receptor are known: GABAA in which the receptor is part of a ligand-gated ion channel complex, and GABAB metabotropic receptors, which are G protein-coupled receptors that open or close ion channels via intermediaries (G proteins).

Neurons that produce GABA as their output are called GABAergic neurons, and have chiefly inhibitory action at receptors in the adult vertebrate. Medium Spiny Cells are a typical example of inhibitory CNS GABAergic cells. In contrast, GABA exhibits excitatory actions in insects, mediating muscle activation at synapses between nerves and muscle cells, and also the stimulation of certain glands. In mammals, some GABAergic neurons, such as chandelier cells, are also able to excite their glutamatergic counterparts.[2]
GABAA receptors are chloride channels; that is, when activated by GABA, they allow the flow of chloride ions across the membrane of the cell. Whether this chloride flow is excitatory/depolarizing (makes the voltage across the cell's membrane less negative), shunting (has no effect on the cell's membrane) or inhibitory/hyperpolarizing (makes the cell's membrane more negative) depends on the direction of the flow of chloride. When net chloride flows out of the cell, GABA is excitatory or depolarizing; when the net chloride flows into the cell, GABA is inhibitory or hyperpolarizing. When the net flow of chloride is close to zero, the action of GABA is shunting. Shunting inhibition has no direct effect on the membrane potential of the cell, however it minimises the effect of any coincident synaptic input essentially by reducing the electrical resistance of the cell's membrane (essentially equivalent to Ohm's law). A developmental switch in the molecular machinery controlling concentration of chloride inside the cell and hence the direction of this ion flow, is responsible for the changes in the functional role of GABA between the neonatal and adult stages. That is to say, GABA's role changes from excitatory to inhibitory as the brain develops into adulthood.[3]
Development
In hippocampus and neocortex of the mammalian brain, GABA has primarily excitatory effects early in development, and is in fact the major excitatory neurotransmitter in many regions of the brain before the maturation of glutamate synapses - See developing cortex.[3]
In the developmental stages preceding the formation of synaptic contacts, GABA is synthesized by neurons and acts both as an autocrine (acting on the same cell) and paracrine (acting on nearby cells) signalling mediator.[4][5]
GABA regulates the proliferation of neural progenitor cells[6][7] the migration[8] and differentiation[9][10] the elongation of neurites[11] and the formation of synapses.[12]
GABA also regulates the growth of embryonic and neural stem cells. GABA can influence the development of neural progenitor cells via brain-derived neurotrophic factor (BDNF) expression.[13] GABA activates the GABAA receptor, causing cell cycle arrest in the S-phase, limiting growth.[14]
Beyond the nervous system

GABAergic mechanisms have been demonstrated in various peripheral tissues and organs including, but not restricted to the intestine, stomach, pancreas, Fallopian tube, uterus, ovary, testis, kidney, urinary bladder, lung and liver.[16]
In 2007, an excitatory GABAergic system was described in the airway epithelium. The system activates following exposure to allergens and may participate in the mechanisms of asthma.[17] GABAergic systems have also been found in the testis[18] and in the eye lens.[19]
Structure and conformation
GABA is found mostly as a zwitterion, that is, with the carboxy group deprotonated and the amino group protonated. Its conformation depends on its environment. In the gas phase, a highly folded conformation is strongly favored because of the electrostatic attraction between the two functional groups. The stabilization is about 50 kcal/mol, according to quantum chemistry calculations. In the solid state, a more extended conformation is found, with a trans conformation at the amino end and a gauche conformation at the carboxyl end. This is due to the packing interactions with the neighboring molecules. In solution, five different conformations, some folded and some extended are found as a result of solvation effects. The conformational flexibility of GABA is important for its biological function, as it has been found to bind to different receptors with different conformations. Many GABA analogues with pharmaceutical applications have more rigid structures in order to control the binding better.[20][21]
History
Gamma-aminobutyric acid was first synthesized in 1883, and was first known only as a plant and microbe metabolic product. In 1950, however, GABA was discovered to be an integral part of the mammalian central nervous system.[22]
Synthesis
Since GABA doesn't penetrate the blood brain barrier, GABA is therefore synthesized in vivo. It's synthesized from glutamate using the enzyme L-glutamic acid decarboxylase and pyridoxal phosphate (which is the active form of vitamin B6) as a cofactor via a metabolic pathway called the GABA shunt. This process converts glutamate, the principal excitatory neurotransmitter, into the principal inhibitory neurotransmitter (GABA).[23][24]
Pharmacology
Drugs that act as agonists of GABA receptors (known as GABA analogues or GABAergic drugs) or increase the available amount of GABA typically have relaxing, anti-anxiety and anti-convulsive effects.[25] Many of the substances below are known to cause anterograde amnesia and retrograde amnesia.
In general, GABA does not cross the blood-brain barrier,[26] although certain areas of the brain which have no effective blood brain barrier, such as the periventricular nucleus, can be reached by drugs such as systematically injected GABA.[27] At least one study suggests that orally administered GABA increases the amount of Human Growth Hormone.[28] GABA directly injected to the brain has been reported to have both stimulatory and inhibitory effects on the production of growth hormone, depending on the physiology of the individual.[27]
GABAergic Drugs
- GABAA receptor ligands
- Agonists/Positive allosteric modulators: alcohol[29][30][31], barbiturates, benzodiazepines, carisoprodol, etomidate, glutethimide, L-theanine, kava, methaqualone, muscimol, neuroactive steroids, nonbenzodiazepines, propofol, scullcap, valerian, volatile/inhaled anaesthetics.
- Antagonists/Negative allosteric modulators: bicuculline, cicutoxin, flumazenil, furosemide, gabazine, oenanthotoxin, picrotoxin, Ro15-4513, thujone.
- GABAB Receptor Ligands
- GABA reuptake inhibitors: deramciclane, hyperforin, tiagabine.
- GABA-transaminase inhibitors: gabaculine, phenelzine, valproate, vigabatrin, Lemon balm (Melissa officinalis) [33].
- GABA analogues: pregabalin, gabapentin.
- Others: GABA (itself), L-glutamine, picamilon, progabide, tetanospasmin.
See also
- Spasticity
- Spastic diplegia, a GABA deficiency neuromuscular neuropathology
References
- ↑ Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H (2002). "GABA and GABA receptors in the central nervous system and other organs". Int. Rev. Cytol. 213: 1–47. doi:10.1016/S0074-7696(02)13011-7. PMID 11837891.
- ↑ Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G (January 2006). "Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits". Science 311 (5758): 233–5. doi:10.1126/science.1121325. PMID 16410524.
- ↑ 3.0 3.1 Li K, Xu E (June 2008). "The role and the mechanism of gamma-aminobutyric acid during central nervous system development". Neurosci Bull 24 (3): 195–200. doi:10.1007/s12264-008-0109-3. PMID 18500393.
- ↑ Purves D, Fitzpatrick D, Hall WC, Augustine GJ, Lamantia A-S (2007). Neuroscience (4th ed.). Sunderland, Mass: Sinauer. pp. 135, box 6D. ISBN 0-87893-697-1.
- ↑ Jelitai M, Madarasz E (2005). "The role of GABA in the early neuronal development". Int. Rev. Neurobiol. 71: 27–62. doi:10.1016/S0074-7742(05)71002-3. PMID 16512345. http://www.iem.cas.cz/Data/files/pdf/neuroscience/2004/jelitai-2004.pdf.
- ↑ LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR (December 1995). "GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis". Neuron 15 (6): 1287–98. doi:10.1016/0896-6273(95)90008-X. PMID 8845153.
- ↑ Haydar TF, Wang F, Schwartz ML, Rakic P (August 2000). "Differential modulation of proliferation in the neocortical ventricular and subventricular zones". J. Neurosci. 20 (15): 5764–74. PMID 10908617. http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=10908617.
- ↑ Behar TN, Schaffner AE, Scott CA, O'Connell C, Barker JL (August 1998). "Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus". J. Neurosci. 18 (16): 6378–87. PMID 9698329. http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=9698329.
- ↑ Barbin G, Pollard H, Gaïarsa JL, Ben-Ari Y (April 1993). "Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons". Neurosci. Lett. 152 (1-2): 150–4. doi:10.1016/0304-3940(93)90505-F. PMID 8390627.
- ↑ Ganguly K, Schinder AF, Wong ST, Poo M (May 2001). "GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition". Cell 105 (4): 521–32. doi:10.1016/S0092-8674(01)00341-5. PMID 11371348.
- ↑ Maric D, Liu QY, Maric I, Chaudry S, Chang YH, Smith SV, Sieghart W, Fritschy JM, Barker JL (April 2001). "GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl- channels". J. Neurosci. 21 (7): 2343–60. PMID 11264309. http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=11264309.
- ↑ Ben-Ari Y (September 2002). "Excitatory actions of gaba during development: the nature of the nurture". Nat. Rev. Neurosci. 3 (9): 728–39. doi:10.1038/nrn920. PMID 12209121.
- ↑ Obrietan K, Gao XB, Van Den Pol AN (August 2002). "Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons". J. Neurophysiol. 88 (2): 1005–15. PMID 12163549. http://jn.physiology.org/cgi/pmidlookup?view=long&pmid=12163549.
- ↑ Wang DD, Kriegstein AR, Ben-Ari Y (2008). "GABA Regulates Stem Cell Proliferation before Nervous System Formation". Epilepsy currents / American Epilepsy Society 8 (5): 137–9. doi:10.1111/j.1535-7511.2008.00270.x. PMID 18852839.
- ↑ Popp A, Urbach A, Witte OW, Frahm C (2009). "Adult and embryonic GAD transcripts are spatiotemporally regulated during postnatal development in the rat brain". PLoS ONE 4 (2): e4371. doi:10.1371/journal.pone.0004371. PMID 19190758. PMC 2629816. http://dx.plos.org/10.1371/journal.pone.0004371.
- ↑ Erdö SL, Wolff JR (1990). "gamma-Aminobutyric acid outside the mammalian brain". J. Neurochem. 54 (2): 363–72. doi:10.1111/j.1471-4159.1990.tb01882.x. PMID 2405103.
- ↑ PMID 17589520 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Payne, Anita H.; Matthew H. Hardy (2007). The Leydig cell in health and disease. Humana Press. ISBN 1588297543, ISBN 9781588297549.
- ↑ PMID 17969168 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Devashis Majumdar and Sephali Guha.(1988). "Conformation, electrostatic potential and pharmacophoric pattern of GABA (gamma-aminobutyric acid) and several GABA inhibitors." Journal of Molecular Structure: THEOCHEM 180: 125-140. doi:10.1016/0166-1280(88)80084-8
- ↑ Anne-Marie Sapse. Molecular Orbital Calculations for Amino Acids and Peptides. Birkhäuser, 2000. ISBN 0817638938.
- ↑ Roth, Robert J.; Cooper, Jack R.; Bloom, Floyd E. (2003). The Biochemical Basis of Neuropharmacology. Oxford [Oxfordshire]: Oxford University Press. pp. 416 pages. ISBN 0-19-514008-7.
- ↑ Petroff OA (December 2002). "GABA and glutamate in the human brain". Neuroscientist 8 (6): 562–73. doi:10.1177/1073858402238515. PMID 12467378. http://nro.sagepub.com/cgi/pmidlookup?view=long&pmid=12467378.
- ↑ Schousboe A, Waagepetersen HS (2007). "GABA: homeostatic and pharmacological aspects". Prog. Brain Res. 160: 9–19. doi:10.1016/S0079-6123(06)60002-2. PMID 17499106.
- ↑ Foster AC, Kemp JA (February 2006). "Glutamate- and GABA-based CNS therapeutics". Curr Opin Pharmacol 6 (1): 7–17. doi:10.1016/j.coph.2005.11.005. PMID 16377242.
- ↑ Kuriyama K, Sze PY (January 1971). "Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals". Neuropharmacology 10 (1): 103–8. doi:10.1016/0028-3908(71)90013-X. PMID 5569303.
- ↑ 27.0 27.1 Müller EE, Locatelli V, Cocchi D (April 1999). "Neuroendocrine control of growth hormone secretion". Physiol. Rev. 79 (2): 511–607. PMID 10221989. Free full-text.
- ↑ Powers ME, Yarrow JF, McCoy SC, Borst SE (January 2008). "Growth hormone isoform responses to GABA ingestion at rest and after exercise". Medicine and science in sports and exercise 40 (1): 104–10. doi:10.1249/mss.0b013e318158b518. ISSN 1530-031. PMID 18091016. http://www.ncbi.nlm.nih.gov/pubmed/18091016.
- ↑ Dzitoyeva S, Dimitrijevic N, Manev H (2003). "Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5485–90. doi:10.1073/pnas.0830111100. PMID 12692303.
- ↑ Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL (1997). "Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors". Nature 389 (6649): 385–9. doi:10.1038/38738. PMID 9311780.
- ↑ Boehm SL, Ponomarev I, Blednov YA, Harris RA (2006). "From gene to behavior and back again: new perspectives on GABAAreceptor subunit selectivity of alcohol actions". Adv. Pharmacol. 54: 171–203. doi:10.1016/j.bcp.2004.07.023. PMID 17175815.
- ↑ Dimitrijevic N, Dzitoyeva S, Satta R, Imbesi M, Yildiz S, Manev H (2005). "Drosophila GABAB receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB)". Eur. J. Pharmacol. 519 (3): 246–52. doi:10.1016/j.ejphar.2005.07.016. PMID 16129424.
- ↑ "Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity". http://www.ncbi.nlm.nih.gov/pubmed/19165747. Retrieved 2010-03-08.
External links
- Lydiard B, Pollack MH, Ketter TA, Kisch E, Hettema JM (2001-10-26). "GABA". Continuing Medical Education. School of Medicine, Virginia Commonwealth University, Medical College of Virginia Campus (VCU), Richmond, VA. http://www.vcu-cme.org/gaba/overview.html. Retrieved 2008-06-20. "The role of GABA in the pathogenesis and treatment of anxiety and other neuropsychiatric disorders"
- Scholarpedia article on GABA
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||