Flavonoid
Flavonoids (or bioflavonoids), also collectively known as Vitamin P and citrin[1], are a class of plant secondary metabolites. According to the IUPAC nomenclature,[2] they can be classified into:
- flavonoids, derived from 2-phenylchromen-4-one (2-phenyl-1,4-benzopyrone) structure (examples: quercetin, rutin).
- isoflavonoids, derived from 3-phenylchromen-4-one (3-phenyl-1,4-benzopyrone) structure
- neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure.
The three flavonoid classes above are all ketone-containing compounds, and as such, are flavonoids and flavonols. This class was the first to be termed "bioflavonoids." The terms flavonoid and bioflavonoid have also been more loosely used to describe non-ketone polyhydroxy polyphenol compounds which are more specifically termed flavanoids, flavan-3-ols, or catechins (although catechins are actually a subgroup of flavanoids).
Contents |
Biosynthesis
Biological roles
Flavonoids are widely distributed in plants fulfilling many functions.
Flavonoids are the most important plant pigments for flower coloration producing yellow or red/blue pigmentation in petals designed to attract pollinator animals.
Flavonoids secreted by the root of their host plant help Rhizobia in the infection stage of their symbiotic relationship with legumes like peas, beans, clover, and soy. Rhizobia living in soil are able to sense the flavonoids and this triggers the secretion of Nod factors, which in turn are recognized by the host plant and can lead to root hair deformation and several cellular responses such as ion fluxes and the formation of a root nodule.
They also protect plants from attacks by microbes, fungi[3] and insects.
Potential for biological activity
Flavonoids (specifically flavanoids such as the catechins) are "the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants".[4] Flavonols, the original bioflavonoids such as quercetin, are also found ubiquitously, but in lesser quantities. Both sets of compounds have evidence of health-modulating effects in animals which eat them.
The widespread distribution of flavonoids, their variety and their relatively low toxicity compared to other active plant compounds (for instance alkaloids) mean that many animals, including humans, ingest significant quantities in their diet. Resulting from experimental evidence that they may modify allergens, viruses, and carcinogens, flavonoids have potential to be biological "response modifiers", such as anti-allergic, anti-inflammatory,[5] anti-microbial[6] and anti-cancer activities shown from in vitro studies.[7]
Antioxidant activity in vitro
Flavonoids (both flavonols and flavanols) are most commonly known for their antioxidant activity in vitro.
Consumers and food manufacturers have become interested in flavonoids for their possible medicinal properties, especially their putative role in prevention of cancers and cardiovascular diseases. Although physiological evidence is not yet established, the beneficial effects of fruits, vegetables, tea, and red wine have sometimes been attributed to flavonoid compounds rather than to known micronutrients, such as vitamins and dietary minerals.[8]
Alternatively, research conducted at the Linus Pauling Institute and evaluated by the European Food Safety Authority indicates that, following dietary intake, flavonoids themselves are of little or no direct antioxidant value.[9][10] As body conditions are unlike controlled test tube conditions, flavonoids and other polyphenols are poorly absorbed (less than 5%), with most of what is absorbed being quickly metabolized and excreted. The increase in antioxidant capacity of blood seen after the consumption of flavonoid-rich foods is not caused directly by flavonoids themselves, but most likely is due to increased uric acid levels that result from metabolism of flavonoids.[11] According to Frei, "we can now follow the activity of flavonoids in the body, and one thing that is clear is that the body sees them as foreign compounds and is trying to get rid of them."
Other potential health benefits
Cancer
Physiological processing of unwanted flavonoid compounds induces so-called Phase II enzymes that also help to eliminate mutagens and carcinogens, and therefore may be of value in cancer prevention. Flavonoids could also induce mechanisms that may kill cancer cells and inhibit tumor invasion.[11] UCLA cancer researchers have found that study participants who ate foods containing certain flavonoids, such as catechins found in strawberries and green and black teas; kaempferol from brussel sprouts and apples; and quercetin from beans, onions and apples, may have reduced risk of obtaining lung cancer.[12]
Research also indicated that only small amounts of flavonoids may be needed for possible benefits. Taking large dietary supplements likely provides no extra benefit and may pose risks. However, certainty of neither a benefit nor a risk has been proven yet in large-scale human intervention trials.[11]
Diarrhea
A study done at Children's Hospital & Research Center Oakland, in collaboration with scientists at Heinrich Heine University in Germany, has shown that epicatechin, quercetin and luteolin can inhibit the development of fluids that result in diarrhea by targeting the intestinal cystic fibrosis transmembrane conductance regulator Cl– transport inhibiting cAMP-stimulated Cl– secretion in the intestine.[13]
Capillary stabilizing agents
Bioflavonoids like rutin, monoxerutin, diosmin, troxerutin and hidrosmin have potential vasoprotective proprieties still under experimental evaluation.
Important flavonoids
Quercetin

Quercetin, a flavonoid and more specifically a flavonol, is the aglycone form of other flavonoid glycosides, such as rutin and quercitrin, found in citrus fruit, buckwheat and onions. Quercetin forms the glycosides, quercitrin and rutin, together with rhamnose and rutinose, respectively.
Although there is preliminary clinical evidence that asthma, lung cancer and breast cancer are lower among people consuming higher dietary levels of quercetin,[14] the consensus of scientists, regulatory authorities such as the FDA, and patient support organizations like the American Cancer Society is that no physiological role exists, stating that dietary quercetin "is unlikely to cause any major problems or benefits."[15]
Epicatechin

Epicatechin may improve blood flow and has potential for cardiac health. Cocoa, the major ingredient of dark chocolate, contains relatively high amounts of epicatechin and has been found to have nearly twice the antioxidant content of red wine and up to three times that of green tea in vitro.[16][17] In the test outlined above, it appears the potential antioxidant effects in vivo are minimal as the antioxidants are rapidly excreted from the body.
Important dietary sources
Good sources of flavonoids include all citrus fruits, berries, ginkgo biloba, onions[18][19], parsley[20], (particularly red onion[21]) pulses[22], tea (especially white and green tea), red wine, seabuckthorn, and dark chocolate (with a cocoa content of seventy percent or greater).
Citrus
The citrus bioflavonoids include hesperidin (a glycoside of the flavanone hesperetin), quercitrin, rutin (two glycosides of the flavonol quercetin), and the flavone tangeritin. In addition to possessing in vitro antioxidant activity and an ability to increase intracellular levels of vitamin C, rutin and hesperidin may have beneficial effects on capillary permeability and blood flow. They also exhibit anti-allergy and anti-inflammatory benefits of quercetin from in vitro studies. Quercetin can also inhibit reverse transcriptase, part of the replication process of retroviruses.[23] The therapeutic relevance of this inhibition has not been established. Hydroxyethylrutosides (HER) have potential for use in the treatment of abnormal capillary permeability, bruising, hemorrhoids, and varicose veins.
Tea

Green tea flavonoids are potent antioxidant compounds in vitro, with potential to reduce incidence of cancer [24][25] and heart disease. The major flavonoids in green tea are kaempferol and catechins (catechin, epicatechin, epicatechin gallate (ECG), and epigallocatechin gallate (EGCG)).
In producing teas such as oolong tea and black tea, the leaves are allowed to oxidize, during which enzymes present in the tea convert some or all of the catechins to larger molecules. However, green tea is produced by steaming the fresh-cut tea leaves, which deactivates these enzymes, and oxidation does not significantly occur. White tea is the least processed of teas and is shown to present the highest amount of catechins known to occur in Camellia sinensis.
Wine
Grape skins contain significant amounts of flavonoids as well as other polyphenols[26]. Both red and white wine contain flavonoids; however, since red wine is produced by fermentation in the presence of the grape skins, red wine has been observed to contain higher levels of flavonoids, and other polyphenolics such as resveratrol.
Dark chocolate
Flavonoids exist naturally in cacao, but because they can be bitter, they are often removed from chocolate, even dark chocolate.[27] Although flavonoids are present in milk chocolate, milk may interfere with their absorption.[28]
Subgroups
Over 5000 naturally occurring flavonoids have been characterized from various plants. They have been classified according to their chemical structure, and are usually subdivided into the following subgroups (for further reading see [29]):
Flavones
Flavones are divided into four groups:[30]
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
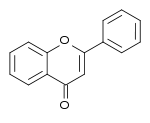
| Flavone | 2-phenylchromen-4-one | ✗ | ✗ |  |
Luteolin, Apigenin, Tangeritin |
| Flavonol or 3-hydroxyflavone |
3-hydroxy-2-phenylchromen-4-one | ✓ | ✗ |  |
Quercetin, Kaempferol, Myricetin, Fisetin, Isorhamnetin, Pachypodol, Rhamnazin |
| Flavanone | 2,3-dihydro-2-phenylchromen-4-one | ✗ | ✓ |  |
Hesperetin, Naringenin, Eriodictyol, Homoeriodictyol |
| Flavanonol or 3-Hydroxyflavanone or 2,3-dihydroflavonol |
3-hydroxy-2,3-dihydro-2-phenylchromen-4-one | ✓ | ✓ |  |
Taxifolin (or Dihydroquercetin), Dihydrokaempferol |
Isoflavones
- Isoflavones
- Isoflavones use the 3-phenylchromen-4-one skeleton (with no hydroxyl group substitution on carbon at position 2).
- Examples: Genistein, Daidzein, Glycitein
Flavan-3-ols, Flavan-4-ols, Flavan-3,4-diols, and proanthocyanidins
Derivatives of flavan.
| Skeleton | Name |
|---|---|
| Flavan-3-ol | |
| Flavan-4-ol | |
| Flavan-3,4-diol (leucoanthocyanidin) |
- Flavan-3-ols (also known as flavanols) and Proanthocyanidins
- Flavan-3-ols use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton.
- Catechins (Catechin (C), Gallocatechin (GC), Catechin 3-gallate (Cg), Gallocatechin 3-gallate (GCg)), Epicatechins (Epicatechin (EC), Epigallocatechin (EGC), Epicatechin 3-gallate (ECg), Epigallocatechin 3-gallate (EGCg))
- Proanthocyanidins are dimers, trimers, oligomers, or polymers of the flavanols.
- Flavan-3-ols use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton.
Anthocyanidins
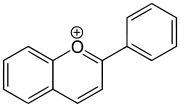
- Anthocyanidins
- Anthocyanidins are the aglycones of anthocyanins. Anthocyanidins use the flavylium (2-phenylchromenylium) ion skeleton
- Examples: Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin
Availability through microorganisms
Several recent research articles have demonstrated the efficient production of flavonoid molecules from genetically-engineered microorganisms[31][32][33].
See also
|
|
References
- ↑ vitamin P, dictionary results
- ↑ Flavonoids (isoflavonoids and neoflavonoids)., IUPAC Compendium of Chemical Terminology
- ↑ Galeotti, F; Barile, E; Curir, P; Dolci, M; Lanzotti, V (2008). "Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity". Phytochemistry Letters 1: 44. doi:10.1016/j.phytol.2007.10.001.
- ↑ Spencer, Jeremy P. E. (2008). "Flavonoids: modulators of brain function?". British Journal of Nutrition 99: ES60–77. doi:10.1017/S0007114508965776. PMID 18503736.
- ↑ "Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer". Yamamoto and Gaynor 107 (2): 135 -- Journal of Clinical Investigation. http://www.jci.org/cgi/content/full/107/2/135?ijkey=a1e09ce2dbca283cec170598f2410b15d5f4304f&keytype2=tf_ipsecsha.
- ↑ Cushnie TPT, Lamb AJ (2005). "Antimicrobial activity of flavonoids". International Journal of Antimicrobial Agents 26 (5): 343–356. doi:10.1016/j.ijantimicag.2005.09.002. PMID 16323269.
- ↑ de Sousa RR, Queiroz KC, Souza AC, Gurgueira SA, Augusto AC, Miranda MA, Peppelenbosch MP, Ferreira CV, Aoyama H. (2007). "Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin". J Enzyme Inhib Med Chem 22 (4): 439–444. doi:10.1080/14756360601162063. PMID 17847710.
- ↑ Félicien Breton (2008). "Health benefits of oligomeric proanthocyanidins". http://www.frenchscout.com/polyphenols#procyanidins.
- ↑ Lotito SB, Frei B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon?". Free Radic. Biol. Med. 41 (12): 1727–46. doi:10.1016/j.freeradbiomed.2006.04.033. PMID 17157175.
- ↑ Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061, EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)2, 3 European Food Safety Authority (EFSA), Parma, Italy, EFSA Journal 2010; 8(2):1489
- ↑ 11.0 11.1 11.2 "Studies force new view on biology of flavonoids", by David Stauth, EurekAlert!. Adapted from a news release issued by Oregon State University. URL accessed
- ↑ UCLA news May 2008 - Fruits, vegetables, teas may protect smokers from lung cancer
- ↑ Schuier M, Sies H, Illek B, Fischer H (1 October 2005). "Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia". J. Nutr. 135 (10): 2320–5. PMID 16177189. http://jn.nutrition.org/cgi/reprint/135/10/2320.
- ↑ Paul Knekt, Jorma Kumpulainen, Ritva Järvinen, Harri Rissanen, Markku Heliövaara, Antti Reunanen, Timo Hakulinen, and Arpo Aromaa (September 2002). "Flavonoid intake and risk of chronic diseases". Am J Clin Nutr 76 (3): 560–8. PMID 12198000.
- ↑ American Cancer Society, Quercetin
- ↑ Lee KW, Kim YJ, Lee HJ, Lee CY (December 2003). "Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine". J. Agric. Food Chem. 51 (25): 7292–5. doi:10.1021/jf0344385. PMID 14640573.
- ↑ "Cocoa nutrient for 'lethal ills'". BBC News. http://news.bbc.co.uk/2/hi/health/.stm.
- ↑ Tsushida T., Suzuki, M. (1996) Content of flavonol glucosides and some properties of enzymes metabolizing the glucosides in onion. J. Jap. Soc. Food Sci. Technol., 43, 642-649.
- ↑ Slimestad R, Fossen T, Vågen IM (December 2007). "Onions: a source of unique dietary flavonoids". J. Agric. Food Chem. 55 (25): 10067–80. doi:10.1021/jf0712503. PMID 17997520.
- ↑ Justesen U, Knuthsen P (2001). "Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes". Food Chem. 73: 245–50. doi:10.1016/S0308-8146(01)00114-5.
- ↑ Marotti, M.; Piccaglia, R. (2002). "Characterization of Flavonoids in Different Cultivars of Onion (Allium cepa L.)". Journal of Food Science 67: 1229. doi:10.1111/j.1365-2621.2002.tb09482.x.
- ↑ Ewald C, Fjelkner-Modig S, Johansson K, Sjöholm I, Åkesson B (1999). "Effect of processing on major flavonoids in processed onions, green beans, and peas". Food Chem. 64: 231–5. doi:10.1016/S0308-8146(98)00136-8.
- ↑ Spedding G, Ratty A, Middleton E (September 1989). "Inhibition of reverse transcriptases by flavonoids". Antiviral Res. 12 (2): 99–110. doi:10.1016/0166-3542(89)90073-9. PMID 2480745.
- ↑ Sartippour MR, Pietras R, Marquez-Garban DC, et al. (December 2006). "The combination of green tea and tamoxifen is effective against breast cancer". Carcinogenesis 27 (12): 2424–33. doi:10.1093/carcin/bgl066. PMID 16785249. http://carcin.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=16785249.
- ↑ BBC news - 17 March 2009 - green tea may have the power to ward off breast cancer
- ↑ Kennedy JA, Matthews MA, Waterhouse AL (2002). "Effect of Maturity and Vine Water Status on Grape Skin and Wine Flavonoids". Am. J. Enol. Vitic. 53 (4): 268–74. http://www.ajevonline.org/cgi/content/abstract/53/4/268.
- ↑ The Lancet, (December 2007). "The devil in the dark chocolate". Lancet 370 (9605): 2070. doi:10.1016/S0140-6736(07)61873-X. PMID 18156011. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(07)61873-X.
- ↑ Serafini et al; Bugianesi, Rossana; Maiani, Giuseppe; Valtuena, Silvia; De Santis, Simone; Crozier, Alan (August 2003). "Plasma antioxidants from chocolate". Nature 424 (6952): 1013. doi:10.1038/4241013a. PMID 12944955. http://www.nature.com/nature/journal/v424/n6952/full/4241013a.html.
- ↑ Ververidis Filippos, F; Trantas Emmanouil, Douglas Carl, Vollmer Guenter, Kretzschmar Georg, Panopoulos Nickolas (October 2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health". Biotechnology Journal 2 (10): 1214. doi:10.1002/biot.200700084. PMID 17935117.
- ↑ Phenolics:figure 4
- ↑ Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (May 2003). "Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster". Appl. Environ. Microbiol. 69 (5): 2699–706. doi:10.1128/AEM.69.5.2699-2706.2003. PMID 12732539. PMC 154558. http://aem.asm.org/cgi/pmidlookup?view=long&pmid=12732539.
- ↑ Trantas Emmanouil, E; Panopoulos Nickolas, Ververidis Filippos (2009). "Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae". Metabolic Engineering 11 (6): 355–366. doi:10.1016/j.ymben.2009.07.004. PMID 19631278.
- ↑ Ververidis Filippos, F; Trantas Emmanouil, Douglas Carl, Vollmer Guenter, Kretzschmar Georg, Panopoulos Nickolas (October 2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part II: Reconstruction of multienzyme pathways in plants and microbes". Biotechnology Journal 2 (10): 1235. doi:10.1002/biot.200700184. PMID 17935118.
External links
- Instant insight considering the effect of flavonoids on memory and learning from the Royal Society of Chemistry
- USDA Database of Flavonoid content of food (pdf)
- Flavonoids (chemistry)
- Cornell news on Cocoa
- A Dark Chocolate a Day Keeps the Doctor Away
- Antioxidant in Green Tea may fight Alzheimer's-EGCG
- Yamamoto Y, Gaynor RB (January 2001). "Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer". J. Clin. Invest. 107 (2): 135–42. doi:10.1172/JCI11914. PMID 11160126. PMC 199180. http://www.jci.org/cgi/content/full/107/2/135.
- Micronutrient Information Center - Flavonoids
|
||||||||||||||||||||
|
|||||
|
||||||||||||||