Enol
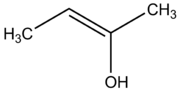
Enols (also known as alkenols) are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates. A reductone is a compound that has an enediol structure with an adjacent carbonyl-group.
The C=C double bond with adjacent alcohol gives enols and enediols their chemical characteristics, by which they present keto-enol tautomerism. In keto-enol tautomerism, enols interconvert with ketones or aldehydes.
The words enol and alkenol are portmanteaux of the words "alkene" (or just -ene, the suffix given to C=C double bonded alkenes) and "alcohol" (which represents the enol's hydroxyl group).
Contents |
Tautomerism
Left the enol form
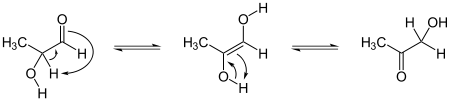
Enols interconvert with carbonyl compounds that have an α-hydrogen, like ketones and aldehydes. The compound is deprotonated on one side and protonated on another side, whereas a single bond and a double bond are exchanged. This is called keto-enol tautomerism.
The enol form is usually unstable, does not survive long, and changes into the keto (ketone). This is because oxygen is more electronegative than carbon and thus forms stronger bonds.
Tautomerism in multi-carbonyl compounds


In 1,3-dicarbonyl and 1,3,5-tricarbonyl compounds does the (mono-)enol form predominate. This is due to intramolecular hydrogen bonding and possibly to an easy internal proton transfer. [1]
Thus, at equilibrium, over 99% of propanedial (OHCCH2CHO) molecules exist as the mono-enol. The percentage is lower for 1,3-aldehyde ketones and diketones (acetylacetone, for example, 80 % enol form).
Enolates
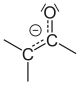
When keto-enol tautomerism occurs the keto or enol is deprotonated and an anion, which is called the enolate, is formed as intermediate. In enolates the anionic charge is delocalized over the oxygen and the carbon. [2] Enolates can exist in quantitative amounts in strictly Brønsted acid free conditions, since they are generally very basic.
| Keto-enol-tautomerism | |||||
|---|---|---|---|---|---|
 |
  |
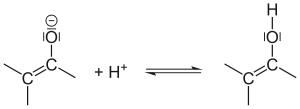 |
|||
| Interconversion between keto form and enolate; deprotonation of the α-C-atom. |
Enolate anion, exhibiting tautomerism. Left the carbanion. |
Interconversion between enolate and enol; protonation of the enolate. |
|||
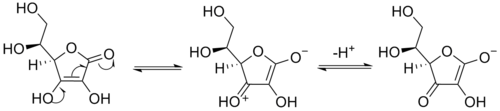
Selective deprotonation in enolate forming
In ketones (a type of carbonyl) with acidic α-hydrogens on either side of the carbonyl carbon, selectivity of deprotonation may be achieved to generate the enolate from the ketone. At low temperatures (-78°C, i.e. dry ice bath), in aprotic solvents, and with bulky non-equilibrating bases (e.g. LDA) the "kinetic" proton may be removed. The "kinetic" proton is the one which is sterically most accessible. Under thermodynamic conditions (higher temperatures, weak base, and protic solvent) equilibrium is established between the ketone and the two possible enolates, the enolate favoured is termed the "thermodynamic" enolate and is favoured because of its lower energy level than the other possible enolate. Thus, by choosing the optimal conditions to generate an enolate, one can increase the yield of the desired product while minimizing formation of undesired products.
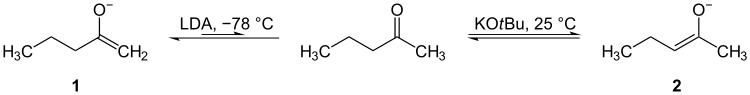
Enediols
Enediols are alkenes with on both sides of the C=C double bond a hydroxyl group. Enediols are reaction intermediates in the Lobry-de Bruyn-van Ekenstein transformation.
Reductones
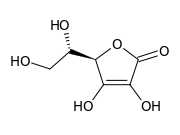
Enediols with a carbonyl group adjacent to the enediol group are called reductones. The enediol structure is stabilized by the resonance, resulting from the tautomerism with the adjacent carbonyl. Therefore, the chemical equilibrium produces mainly the enediol form rather than the keto form. Reductones are strong reducing agents, thus efficacious antioxidants, and fairly strong acids.[3]
Examples of reductones are tartronaldehyde, reductic acid and ascorbic acid.
| Examples of reductones | ||
|---|---|---|
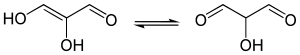 |
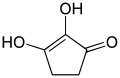 |
 |
| Tartronaldehyde | Reductic acid | Ascorbic acid (Vitamin C) |
Weblinks
References
- ↑ W. Caminati, J.-U. Grabow (2006). "The C2v Structure of Enolic Acetylacetone". Journal of the American Chemical Society 128 (3): 854–857. doi:10.1021/ja055333g.
- ↑ IUPAC Gold Book enolates
- ↑ IUPAC Gold Book reductones