Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible.
Electrolytes commonly exist as solutions of acids, bases or salts. Furthermore, some gases may act as electrolytes under conditions of high temperature or low pressure. Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) and synthetic polymers (e.g., polystyrene sulfonate), termed polyelectrolytes, which contain charged functional group.
Electrolyte solutions are normally formed when a salt is placed into a solvent such as water and the individual components dissociate due to the thermodynamic interactions between solvent and solute molecules, in a process called solvation. For example, when table salt, NaCl, is placed in water, the salt (a solid) dissolves into its component ions, according to the dissociation reaction
- NaCl(s) → Na+(aq) + Cl−(aq)
It is also possible for substances to react with water when they are added to it, producing ions, e.g., carbon dioxide gas dissolves in water to produce a solution which contains hydronium, carbonate, and hydrogen carbonate ions.
Note that molten salts can be electrolytes as well. For instance, when sodium chloride is molten, the liquid conducts electricity.
An electrolyte in a solution may be described as concentrated if it has a high concentration of ions, or dilute if it has a low concentration. If a high proportion of the solute dissociates to form free ions, the electrolyte is strong; if most of the solute does not dissociate, the electrolyte is weak. The properties of electrolytes may be exploited using electrolysis to extract constituent elements and compounds contained within the solution.
Contents |
Physiological importance
In physiology, the primary ions of electrolytes are sodium(Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), hydrogen phosphate (HPO42−), and hydrogen carbonate (HCO3−). The electric charge symbols of plus (+) and minus (−) indicate that the substance in question is ionic in nature and has an imbalanced distribution of electrons, which is the result of chemical dissociation.
All known higher lifeforms require a subtle and complex electrolyte balance between the intracellular and extracellular milieu. In particular, the maintenance of precise osmotic gradients of electrolytes is important. Such gradients affect and regulate the hydration of the body as well as blood pH, and are critical for nerve and muscle function. Various mechanisms exist in living species that keep the concentrations of different electrolytes under tight control.
Both muscle tissue and neurons are considered electric tissues of the body. Muscles and neurons are activated by electrolyte activity between the extracellular fluid or interstitial fluid, and intracellular fluid. Electrolytes may enter or leave the cell membrane through specialized protein structures embedded in the plasma membrane called ion channels. For example, muscle contraction is dependent upon the presence of calcium (Ca2+), sodium (Na+), and potassium (K+). Without sufficient levels of these key electrolytes, muscle weakness or severe muscle contractions may occur.
Electrolyte balance is maintained by oral, or in emergencies, intravenous (IV) intake of electrolyte-containing substances, and is regulated by hormones, generally with the kidneys flushing out excess levels. In humans, electrolyte homeostasis is regulated by hormones such as antidiuretic hormone, aldosterone and parathyroid hormone. Serious electrolyte disturbances, such as dehydration and overhydration, may lead to cardiac and neurological complications and, unless they are rapidly resolved, will result in a medical emergency.
Measurement
Measurement of electrolytes is a commonly performed diagnostic procedure, performed via blood testing with ion selective electrodes or urinalysis by medical technologists. The interpretation of these values is somewhat meaningless without analysis of the clinical history and is often impossible without parallel measurement of renal function. Electrolytes measured most often are sodium and potassium. Chloride levels are rarely measured except for arterial blood gas interpretation since they are inherently linked to sodium levels. One important test conducted on urine is the specific gravity test to determine the occurrence of electrolyte imbalance.
Rehydration
In oral rehydration therapy, electrolyte drinks containing sodium and potassium salts replenish the body's water and electrolyte levels after dehydration caused by exercise, excessive drinking, diaphoresis, diarrhea, vomiting, intoxication or starvation. Athletes exercising in extreme conditions (for three or more hours continuously e.g. marathon or triathlon) who do not consume electrolytes risk dehydration (or hyponatremia).[1]
A simple electrolyte drink can be home-made by using the correct proportions of water, sugar, salt, salt substitute for potassium, and baking soda.[2] However, effective electrolyte replacements should include all electrolytes required by the body, including sodium chloride, potassium, magnesium, and calcium that can be either obtained in a sports drink or a solid electrolyte capsule.[3]
Electrolytes are commonly found in fruit juices, sports drinks, tomato soup and many fruits and vegetables (e.g. potatoes, avocados).
Electrochemistry
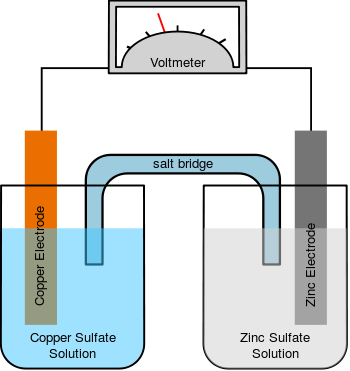
When electrodes are placed in an electrolyte and a voltage is applied, the electrolyte will conduct electricity. Lone electrons normally cannot pass through the electrolyte; instead, a chemical reaction occurs at the cathode consuming electrons from the anode, and another reaction occurs at the anode producing electrons to be taken up by the cathode. As a result, a negative charge cloud develops in the electrolyte around the cathode, and a positive charge develops around the anode. The ions in the electrolyte move to neutralize these charges so that the reactions can continue and the electrons can keep flowing.
For example, in a solution of ordinary table salt (sodium chloride, NaCl) in water, the cathode reaction will be
- 2H2O + 2e− → 2OH− + H2
and hydrogen gas will bubble up; the anode reaction is
- 2NaCl → 2 Na+ + Cl2
and chlorine gas will be liberated. The positively charged sodium ions Na+ will react towards the cathode neutralizing the negative charge of OH− there, and the negatively charged oxide ions O2− will react towards the anode neutralizing the positive charge of H+ there. Without the ions from the electrolyte, the charges around the electrode would slow down continued electron flow; diffusion of H+ and OH− through water to the other electrode takes longer than movement of the much more prevalent salt ions.
In other systems, the electrode reactions can involve the metals of the electrodes as well as the ions of the electrolyte.
Electrolytic conductors are used in electronic devices where the chemical reaction at a metal/electrolyte interface yields useful effects.
- In batteries, two metals with different electron affinities are used as electrodes; electrons flow from one electrode to the other outside of the battery, while inside the battery the circuit is closed by the electrolyte's ions. Here the electrode reactions convert chemical energy to electrical energy.
- In some fuel cells, a solid electrolyte or proton conductor connects the plates electrically while keeping the hydrogen and oxygen fuel gases separated.
- In electroplating tanks, the electrolyte simultaneously deposits metal onto the object to be plated, and electrically connects that object in the circuit.
- In operation-hours gauges, two thin columns of mercury are separated by a small electrolyte-filled gap, and, as charge is passed through the device, the metal dissolves on one side and plates out on the other, causing the visible gap to slowly move along.
- In electrolytic capacitors the chemical effect is used to produce an extremely thin 'dielectric' or insulating coating, while the electrolyte layer behaves as one capacitor plate.
- In some hygrometers the humidity of air is sensed by measuring the conductivity of a nearly dry electrolyte.
- Hot, softened glass is an electrolytic conductor, and some glass manufacturers keep the glass molten by passing a large current through it.
Dry electrolyte
Dry electrolytes are essentially gels in a flexible lattice framework.[4]
See also
- Strong electrolyte
- ITIES (Interface between Two Immiscible Electrolyte Solutions)
References
- ↑ Coso J,Estevez E,Baquero E,Mora-Rodriguez R (2008). "Anaerobic performance when rehydrating with water or commercially available sports drinks during prolonged exercise in the heat". Applied Physiology, Nutrition and Metabolism 33 (2): 290–298. doi:10.1139/H07-188. PMID 18347684.
- ↑ "Rehydration drinks". Webmd.com. 2008-04-28. http://www.webmd.com/hw/health_guide_atoz/str2254.asp?navbar=hw86827. Retrieved 2010-08-20.
- ↑ "Runner's Web and Triathlete's Web, a Running, Track and Field and Triathlon Resource Portal". Runnersweb.com. 2005-08-24. http://www.runnersweb.com/running/rw_news_frameset.html?http://www.runnersweb.com/running/news/rw_news_20060612_ERB_Electrolytes.html. Retrieved 2010-08-20.
- ↑ "The Roll-to-Roll Battery Revolution". Ev World. http://www.evworld.com/article.cfm?storyid=933. Retrieved 2010-08-20.
|
||||||||||||||||