Dinosaur
| Dinosaurs Fossil range: Late Triassic-Late Cretaceous, 230–65.5 Ma Descendant taxon Aves survives to present |
|
|---|---|
 |
|
| Mounted skeletons of Tyrannosaurus (left) and Apatosaurus (right) at the American Museum of Natural History | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Vertebrata |
| Class: | Reptilia |
| Subclass: | Diapsida |
| Infraclass: | Archosauromorpha |
| Superorder: | Dinosauria Owen, 1842 |
| Orders and suborders | |
Dinosaurs are a diverse group of reptiles. They were the dominant terrestrial vertebrates for over 160 million years, from the late Triassic period (about 230 million years ago) until the end of the Cretaceous period (about 65 million years ago), when the Cretaceous–Tertiary extinction event caused the extinction of most dinosaur species, except for some birds. The fossil record indicates that birds evolved from theropod dinosaurs during the Jurassic period, and most paleontologists regard them as the only group of dinosaurs to have survived until the present day.[1]
Dinosaurs are a diverse and varied group of animals; birds, at over 9,000 species, are the most diverse group of vertebrate besides perciform fish.[2] Paleontologists have identified over 500 distinct genera[3] and more than 1,000 different species of non-avian dinosaurs.[4] Dinosaurs are represented on every continent by both extant species and fossil remains.[5] Some dinosaurs are or were herbivorous, others carnivorous. Some have been bipedal, others quadrupedal, and others have been able to shift between these body postures. Many non-avian species developed elaborate skeletal modifications such as bony armor, horns or crests. Avian dinosaurs have been the planet's dominant flying vertebrate since the extinction of the pterosaurs. Although generally known for the large size of some species, most dinosaurs were human-sized or even smaller. Most groups of dinosaurs are known to have built nests and laid eggs.
The term "dinosaur" was coined in 1842 by the English paleontologist Richard Owen, and derives from Greek δεινός (deinos) "terrible, powerful, wondrous" + σαῦρος (sauros) "lizard". Through the first half of the twentieth century, most of the scientific community believed dinosaurs to have been sluggish, unintelligent cold-blooded animals. Most research conducted since the 1970s, however, has indicated that dinosaurs were active animals with elevated metabolisms and numerous adaptations for social interaction.
Since the first dinosaur fossils were recognized in the early nineteenth century, mounted dinosaur skeletons have been major attractions at museums around the world, and dinosaurs have become a part of world culture. They have been featured in best-selling books and films such as Jurassic Park, and new discoveries are regularly covered by the media. The outdated image of dinosaurs as maladapted extinct monsters has led to the word "dinosaur" entering the vernacular to describe anything that is impractically large, slow-moving, obsolete, or bound for extinction.[6]
Contents |
Etymology
The taxon Dinosauria was formally named in 1842 by Sir Richard Owen, who used it to refer to the "distinct tribe or sub-order of Saurian Reptiles" that were then being recognized in England and around the world.[7]:103 The term is derived from the Greek words δεινός (deinos meaning "terrible", "powerful", or "wondrous") and σαῦρος (sauros meaning "lizard" or "reptile").[7]:103[8] Though the taxonomic name has often been interpreted as a reference to dinosaurs' teeth, claws, and other fearsome characteristics, Owen intended it merely to evoke their size and majesty.[9] In colloquial English "dinosaur" is sometimes used to describe an obsolete or unsuccessful thing or person,[10] despite the dinosaurs' 160 million year reign and the global abundance and diversity of their avian descendants: modern-day birds.
Modern definition

Under phylogenetic taxonomy, dinosaurs are usually defined as the group consisting of "Triceratops, Neornithes [modern birds], their most recent common ancestor, and all descendants."[11] It has also been suggested that Dinosauria be defined with respect to the most recent common ancestor of Megalosaurus and Iguanodon, because these were two of the three genera cited by Richard Owen when he recognized the Dinosauria.[12] Both definitions result in the same set of animals being defined as dinosaurs, including theropods (mostly bipedal carnivores), sauropodomorphs (mostly large herbivorous quadrupeds with long necks and tails), ankylosaurians (armored herbivorous quadrupeds), stegosaurians (plated herbivorous quadrupeds), ceratopsians (herbivorous quadrupeds with horns and frills), and ornithopods (bipedal or quadrupedal herbivores including "duck-bills"). These definitions are written to correspond with scientific conceptions of dinosaurs that predate the modern use of phylogenetics. The continuity of meaning is intended to prevent confusion about what the term "dinosaur" means.
There is a wide consensus among paleontologists that birds are the descendants of theropod dinosaurs. Using the strict cladistical definition that all descendants of a single common ancestor must be included in a group for that group to be natural, birds would thus be dinosaurs and dinosaurs are, therefore, not extinct. Birds are classified by most paleontologists as belonging to the subgroup Maniraptora, which are coelurosaurs, which are theropods, which are saurischians, which are dinosaurs.[13]
From the point of view of cladistics, birds are dinosaurs, but in ordinary speech the word "dinosaur" does not include birds. Additionally, referring to dinosaurs that are not birds as "non-avian dinosaurs" is cumbersome. For clarity, this article will use "dinosaur" as a synonym for "non-avian dinosaur". The term "non-avian dinosaur" will be used for emphasis as needed.
General description

Using one of the above definitions, dinosaurs (aside from birds) can be generally described as terrestrial archosaurian reptiles with limbs held erect beneath the body, that existed from the Late Triassic (first appearing in the Carnian faunal stage) to the Late Cretaceous (going extinct at the end of the Maastrichtian).[14] Many prehistoric animals are popularly conceived of as dinosaurs, such as ichthyosaurs, mosasaurs, plesiosaurs, pterosaurs, and Dimetrodon, but are not classified scientifically as dinosaurs. Marine reptiles like ichthyosaurs, mosasaurs, and plesiosaurs were neither terrestrial nor archosaurs; pterosaurs were archosaurs but not terrestrial; and Dimetrodon was a Permian animal more closely related to mammals.[15] Dinosaurs were the dominant terrestrial vertebrates of the Mesozoic, especially the Jurassic and Cretaceous. Other groups of animals were restricted in size and niches; mammals, for example, rarely exceeded the size of a cat, and were generally rodent-sized carnivores of small prey.[16] One notable exception is Repenomamus giganticus, a triconodont weighing between 12 kilograms (26 lb) and 14 kilograms (31 lb) that is known to have eaten small dinosaurs like young Psittacosaurus.[17]
Dinosaurs were an extremely varied group of animals; according to a 2006 study, over 500 dinosaur genera have been identified with certainty so far, and the total number of genera preserved in the fossil record has been estimated at around 1850, nearly 75% of which remain to be discovered.[3] An earlier study predicted that about 3400 dinosaur genera existed, including many which would not have been preserved in the fossil record.[18] As of September 17, 2008, 1047 different species of dinosaurs have been named.[4] Some were herbivorous, others carnivorous. Some dinosaurs were bipeds, some were quadrupeds, and others, such as Ammosaurus and Iguanodon, could walk just as easily on two or four legs. Many had bony armor, or cranial modifications like horns and crests. Although known for large size, many dinosaurs were human-sized or smaller. Dinosaur remains have been found on every continent on Earth, including Antarctica.[5] No dinosaurs are known to have lived in marine or aerial habitats, although it is possible some feathered theropods were flyers. There is also evidence that some spinosaurids had semi-aquatic habits.[19]
Distinguishing anatomical features
While recent discoveries have made it more difficult to present a universally agreed-upon list of dinosaurs' distinguishing features, nearly all dinosaurs discovered so far share certain modifications to the ancestral archosaurian skeleton. Although some later groups of dinosaurs featured further modified versions of these traits, they are considered typical across Dinosauria; the earliest dinosaurs had them and passed them on to all their descendants. Such common features across a taxonomic group are called synapomorphies.
Dinosaur synapomorphies include an elongated crest on the humerus, or upper arm bone, to accommodate the attachment of deltopectoral muscles; a shelf at the rear of the ilium, or main hip bone; a tibia, or shin bone, featuring a broad lower edge and a flange pointing out and to the rear; and an ascending projection on the astragalus, one of the ankle bones, which secures it to the tibia.[11]

A variety of other skeletal features were shared by many dinosaurs. However, because they were either common to other groups of archosaurs or were not present in all early dinosaurs, these features are not considered to be synapomorphies. For example, as diapsid reptiles, dinosaurs ancestrally had two pairs of temporal fenestrae (openings in the skull behind the eyes), and as members of the diapsid group Archosauria, had additional openings in the snout and lower jaw.[20] Additionally, several characteristics once thought to be synapomorphies are now known to have appeared before dinosaurs, or were absent in the earliest dinosaurs and independently evolved by different dinosaur groups. These include an elongated scapula, or shoulder blade; a sacrum composed of three or more fused vertebrae (three are found in some other archosaurs, but only two are found in Herrerasaurus);[11] and an acetabulum, or hip socket, with a hole at the center of its inside surface (closed in Saturnalia, for example).[21] Another difficulty of determining distinctly dinosaurian features is that early dinosaurs and other archosaurs from the Late Triassic are often poorly known and were similar in many ways; these animals have sometimes been misidentified in the literature.[22]

Dinosaurs stood erect in a manner similar to most modern mammals, but distinct from most other reptiles, whose limbs sprawl out to either side.[23] Their posture was due to the development of a laterally facing recess in the pelvis (usually an open socket) and a corresponding inwardly facing distinct head on the femur.[24] Their erect posture enabled dinosaurs to breathe easily while moving, which likely permitted stamina and activity levels that surpassed those of "sprawling" reptiles.[25] Erect limbs probably also helped support the evolution of large size by reducing bending stresses on limbs.[26] Some non-dinosaurian archosaurs, including rauisuchians, also had erect limbs but achieved this by a "pillar erect" configuration of the hip joint, where instead of having a projection from the femur insert on a socket on the hip, the upper pelvic bone was rotated to form an overhanging shelf.[26]
Natural history
Origins and early evolution
For a long time many scientists thought dinosaurs were polyphyletic with multiple groups of unrelated "dinosaurs" evolving due to similar pressures,[27][28][29] but dinosaurs are now known to have formed a single group.[11][30]
Dinosaurs diverged from their archosaur ancestors approximately 230 million years ago during the Middle to Late Triassic period, roughly 20 million years after the Permian–Triassic extinction event wiped out an estimated 95% of all life on Earth.[31][32] Radiometric dating of the rock formation that contained fossils from the early dinosaur genus Eoraptor establishes its presence in the fossil record at this time. Paleontologists believe Eoraptor resembles the common ancestor of all dinosaurs;[33] if this is true, its traits suggest that the first dinosaurs were small, bipedal predators.[34] The discovery of primitive, dinosaur-like ornithodirans such as Marasuchus and Lagerpeton in Argentinian Middle Triassic strata supports this view; analysis of recovered fossils suggests that these animals were indeed small, bipedal predators.
When dinosaurs appeared, terrestrial habitats were occupied by various types of basal archosaurs and therapsids, such as aetosaurs, cynodonts, dicynodonts, ornithosuchids, rauisuchias, and rhynchosaurs. Most of these other animals became extinct in the Triassic, in one of two events. First, at about the boundary between the Carnian and Norian faunal stages (about 215 million years ago), dicynodonts and a variety of basal archosauromorphs, including the prolacertiforms and rhynchosaurs, became extinct. This was followed by the Triassic–Jurassic extinction event (about 200 million years ago), that saw the end of most of the other groups of early archosaurs, like aetosaurs, ornithosuchids, phytosaurs, and rauisuchians. These losses left behind a land fauna of crocodylomorphs, dinosaurs, mammals, pterosaurians, and turtles.[11]

The first few lines of primitive dinosaurs diversified through the Carnian and Norian stages of the Triassic, most likely by occupying the niches of groups that became extinct. Traditionally, dinosaurs were thought to have replaced the variety of other Triassic land animals by proving superior through a long period of competition. This now appears unlikely, for several reasons. Dinosaurs do not show a pattern of steadily increasing in diversity and numbers, as would be predicted if they were competitively replacing other groups; instead, they were very rare through the Carnian, making up only 1–2% of individuals present in faunas. In the Norian, however, after the extinction of several other groups, they became significant components of faunas, representing 50–90% of individuals. Also, what had been viewed as a key adaptation of dinosaurs, their erect stance, is now known to have been present in several contemporaneous groups that were not as successful (aetosaurs, ornithosuchids, rauisuchians, and some groups of crocodylomorphs). Finally, the Late Triassic itself was a time of great upheaval in life, with shifts in plant life, marine life, and climate.[11] Crurotarsans, today represented only by crocodilians but in the Late Triassic also encompassing such now-extinct groups as aetosaurs, phytosaurs, ornithosuchians, and rauisuchians, were actually more diverse in the Late Triassic than dinosaurs, indicating that the survival of dinosaurs had more to do with luck than superiority.[35]
Low diversification in the Cretaceous
Statistical analyses based on raw data suggest that dinosaurs diversified, i.e. the number of species increased, in the Late Cretaceous. However in July 2008 Graeme T. Lloyd et al. argued that this apparent diversification was an illusion caused by sampling bias, because Late Cretaceous rocks have been very heavily studied. Instead, they wrote, dinosaurs underwent only two significant diversifications in the Late Cretaceous, the initial radiations of the euhadrosaurs and ceratopsians. In the Mid Cretaceous, the flowering angiosperm plants became a major part of terrestrial ecosystems, which had previous been dominated by gymnosperms such as conifers. Dinosaur coprolites (fossilized dung) indicate that, while some ate angiosperms, most herbivorous dinosaurs mainly ate gymnosperms. Meanwhile herbivorous insects and mammals diversified rapidly to take advantage of the new type of plant food, while lizards, snakes, crocodilians and birds also diversified at the same time. Lloyd et al. suggest that dinosaurs' failure to diversify as ecosystems were changing doomed them to extinction.[36]
Classification
Dinosaurs (including birds) are archosaurs, like modern crocodilians. Archosaurs' diapsid skulls have two holes, called temporal fenestrae, located where the jaw muscles attach, and an additional antorbital fenestra in front of the eyes. Most reptiles (including birds) are diapsids; mammals, with only one temporal fenestra, are called synapsids; and turtles, with no temporal fenestra, are anapsids. Anatomically, dinosaurs share many other archosaur characteristics, including teeth that grow from sockets rather than as direct extensions of the jawbones. Within the archosaur group, dinosaurs are differentiated most noticeably by their gait. Dinosaur legs extend directly beneath the body, whereas the legs of lizards and crocodilians sprawl out to either side.
Collectively, dinosaurs are usually regarded as a superorder or an unranked clade. They are divided into two orders, Saurischia and Ornithischia, depending upon pelvic structure. Saurischia includes those taxa sharing a more recent common ancestor with birds than with Ornithischia, while Ornithischia includes all taxa sharing a more recent common ancestor with Triceratops than with Saurischia. Saurischians ("lizard-hipped", from the Greek sauros (σαυρος) meaning "lizard" and ischion (ισχιον) meaning "hip joint") retained the hip structure of their ancestors, with a pubis bone directed cranially, or forward.[24] This basic form was modified by rotating the pubis backward to varying degrees in several groups (Herrerasaurus,[37] therizinosauroids,[38] dromaeosaurids,[39] and birds[13]). Saurischia includes the theropods (bipedal and mostly carnivores, except for birds) and sauropodomorphs (long-necked quadrupedal herbivores).
By contrast, ornithischians ("bird-hipped", from the Greek ornitheios (ορνιθειος) meaning "of a bird" and ischion (ισχιον) meaning "hip joint") had a pelvis that superficially resembled a bird's pelvis: the pubis bone was oriented caudally (rear-pointing) Unlike birds, the ornithischian pubis also usually had an additional forward-pointing process. Ornithischia includes a variety of herbivores. (NB: the terms "lizard hip" and "bird hip" are misnomers – birds evolved from dinosaurs with "lizard hips".)
 Saurischian pelvis structure (left side) |
 Tyrannosaurus pelvis (showing saurischian structure – left side) |
 Ornithischian pelvis structure (left side) |
 Edmontosaurus pelvis (showing ornithischian structure – left side) |
The following is a simplified classification of dinosaur families. A more detailed version can be found at List of dinosaur classifications.


- Dinosauria
-
- Saurischia (theropods and sauropods)
-
-
- Coelophysoids (Coelophysis and close relatives)
- Ceratosaurians (Ceratosaurus and abelisaurids – the latter were important Late Cretaceous predators in southern continents)
- Spinosauroids (long bodies; short arms; some with crocodile-like skulls and bony "sails" on their backs)
- Carnosaurians (Allosaurus and close relatives, like Carcharodontosaurus)
- Coelurosaurians (diverse, with a range of body sizes and niches)
-
- Tyrannosauroids (small to gigantic, often with reduced forelimbs)
- Ornithomimosaurians ("ostrich-mimics"; mostly toothless; carnivores to possible herbivores)
- Therizinosauroids (bipedal herbivores with large hand claws and small heads)
- Oviraptorosaurians (mostly toothless; their diet and lifestyle are uncertain)
- Dromaeosaurids (popularly known as "raptors"; bird-like carnivores)
- Troodontids (similar to dromaeosaurids, but more lightly built)
- Avialans (flying dinosaurs, including modern birds: the only living dinosaurs)
- Sauropodomorphs (quadrupedal herbivores with small heads, long necks and tails, and elephant-like bodies)
-
- "Prosauropods" (early relatives of sauropods; small to quite large; some possibly omnivorous; bipeds and quadrupeds)
- Sauropods (very large, usually over 15 meters long [49 ft])
-
- Diplodocoids (skulls and tails elongated; teeth typically narrow and pencil-like)
- Macronarians (boxy skulls; spoon-shaped or pencil-shaped teeth)
-
- Brachiosaurids (very long necks; forelimbs longer than hindlimbs)
- Titanosaurians (diverse; stocky, with wide hips; most common in the Late Cretaceous of southern continents)
-
- Ornithischians (diverse bipedal and quadrupedal herbivores)
-
- Heterodontosaurids (meter- or yard-scale herbivores or omnivores with prominent canine teeth)
- Thyreophorans (armored dinosaurs; mostly quadrupeds)
-
- Ankylosaurians (scutes as primary armor; some had club-like tails)
- Stegosaurians (spikes and plates as primary armor)
- Ornithopods (diverse, from meter- or yard-scale bipeds to 12-meter (39 ft) animals that could move as both bipeds and quadrupeds; evolved a method of chewing using skull flexibility and large numbers of teeth)
-
- Hadrosaurids ("duckbilled dinosaurs")
- Pachycephalosaurians ("bone-heads"; bipeds with domed or knobby growth on skulls)
- Ceratopsians (dinosaurs with horns and frills, although most early forms had only the beginnings of these features)
Evolution and paleobiogeography
Dinosaur evolution after the Triassic follows changes in vegetation and the location of continents. In the Late Triassic and Early Jurassic, the continents were connected as the single landmass Pangaea, and there was a worldwide dinosaur fauna mostly composed of coelophysoid carnivores and prosauropod herbivores.[40] Gymnosperm plants (particularly conifers), a potential food source, radiated in the Late Triassic. Prosauropods did not have sophisticated mechanisms for processing food in the mouth, and so must have employed other means of breaking down food farther along the digestive tract.[41] The general homogeneity of dinosaurian faunas continued into the Middle and Late Jurassic, where most localities had predators consisting of ceratosaurians, spinosauroids, and carnosaurians, and herbivores consisting of stegosaurian ornithischians and large sauropods. Examples of this include the Morrison Formation of North America and Tendaguru Beds of Tanzania. Dinosaurs in China show some differences, with specialized sinraptorid theropods and unusual, long-necked sauropods like Mamenchisaurus.[40] Ankylosaurians and ornithopods were also becoming more common, but prosauropods had become extinct. Conifers and pteridophytes were the most common plants. Sauropods, like the earlier prosauropods, were not oral processors, but ornithischians were evolving various means of dealing with food in the mouth, including potential cheek-like organs to keep food in the mouth, and jaw motions to grind food.[41] Another notable evolutionary event of the Jurassic was the appearance of true birds, descended from maniraptoran coelurosaurians.[13]

By the Early Cretaceous and the ongoing breakup of Pangaea, dinosaurs were becoming strongly differentiated by landmass. The earliest part of this time saw the spread of ankylosaurians, iguanodontians, and brachiosaurids through Europe, North America, and northern Africa. These were later supplemented or replaced in Africa by large spinosaurid and carcharodontosaurid theropods, and rebbachisaurid and titanosaurian sauropods, also found in South America. In Asia, maniraptoran coelurosaurians like dromaeosaurids, troodontids, and oviraptorosaurians became the common theropods, and ankylosaurids and early ceratopsians like Psittacosaurus became important herbivores. Meanwhile, Australia was home to a fauna of basal ankylosaurians, hypsilophodonts, and iguanodontians.[40] The stegosaurians appear to have gone extinct at some point in the late Early Cretaceous or early Late Cretaceous. A major change in the Early Cretaceous, which would be amplified in the Late Cretaceous, was the evolution of flowering plants. At the same time, several groups of dinosaurian herbivores evolved more sophisticated ways to orally process food. Ceratopsians developed a method of slicing with teeth stacked on each other in batteries, and iguanodontians refined a method of grinding with tooth batteries, taken to its extreme in hadrosaurids.[41] Some sauropods also evolved tooth batteries, best exemplified by the rebbachisaurid Nigersaurus.[42]
There were three general dinosaur faunas in the Late Cretaceous. In the northern continents of North America and Asia, the major theropods were tyrannosaurids and various types of smaller maniraptoran theropods, with a predominantly ornithischian herbivore assemblage of hadrosaurids, ceratopsians, ankylosaurids, and pachycephalosaurians. In the southern continents that had made up the now-splitting Gondwana, abelisaurids were the common theropods, and titanosaurian sauropods the common herbivores. Finally, in Europe, dromaeosaurids, rhabdodontid iguanodontians, nodosaurid ankylosaurians, and titanosaurian sauropods were prevalent.[40] Flowering plants were greatly radiating,[41] with the first grasses appearing by the end of the Cretaceous.[43] Grinding hadrosaurids and shearing ceratopsians became extremely diverse across North America and Asia. Theropods were also radiating as herbivores or omnivores, with therizinosaurians and ornithomimosaurians becoming common.[41]
The Cretaceous–Tertiary extinction event, which occurred approximately 65 million years ago at the end of the Cretaceous period, caused the extinction of all dinosaurs except for the birds. Some other diapsid groups, such as crocodilians, lizards, snakes, sphenodontians, and choristoderans, also survived the event.[44]
Paleobiology
Knowledge about dinosaurs is derived from a variety of fossil and non-fossil records, including fossilized bones, feces, trackways, gastroliths, feathers, impressions of skin, internal organs and soft tissues.[45][46] Many fields of study contribute to our understanding of dinosaurs, including physics (especially biomechanics), chemistry, biology, and the earth sciences (of which paleontology is a sub-discipline). Two topics of particular interest and study have been dinosaur size and behavior.
Size
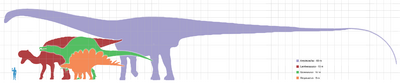
While the evidence is incomplete, it is clear that, as a group, dinosaurs were large. Even by dinosaur standards, the sauropods were gigantic. For much of the dinosaur era, the smallest sauropods were larger than anything else in their habitat, and the largest were an order of magnitude more massive than anything else that has since walked the Earth. Giant prehistoric mammals such as the Indricotherium and the Columbian mammoth were dwarfed by the giant sauropods, and only a handful of modern aquatic animals approach or surpass them in size – most notably the blue whale, which reaches up to 173000 kg (381000 lb) and over 30 meters (100 ft) in length.[47] There are several proposed advantages for the large size of sauropods, including protection from predation, reduction of energy use, and longevity, but it may be that the most important advantage was dietary. Large animals are more efficient at digestion than small animals, because food spends more time in their digestive systems. This also permits them to subsist on food with lower nutritive value than smaller animals. Sauropod remains are mostly found in rock formations interpreted as dry or seasonally dry, and the ability to eat large quantities of low-nutrient browse would have been advantageous in such environments.[48]
Most dinosaurs, however, were much smaller than the giant sauropods. Current evidence suggests that dinosaur average size varied through the Triassic, early Jurassic, late Jurassic and Cretaceous periods.[33] Theropod dinosaurs, when sorted by estimated weight into categories based on order of magnitude, most often fall into the 100 to 1000 kilogram (220 to 2200 lb) category, whereas recent predatory carnivorans peak in the 10 to 100 kilogram (22 to 220 lb) category.[49] The mode of dinosaur body masses is between one and ten metric tonnes.[50] This contrasts sharply with the size of Cenozoic mammals, estimated by the National Museum of Natural History as about 2 to 5 kilograms (5 to 10 lb).[51]
Largest and smallest
Only a tiny percentage of animals ever fossilize, and most of these remain buried in the earth. Few of the specimens that are recovered are complete skeletons, and impressions of skin and other soft tissues are rare. Rebuilding a complete skeleton by comparing the size and morphology of bones to those of similar, better-known species is an inexact art, and reconstructing the muscles and other organs of the living animal is, at best, a process of educated guesswork. As a result, scientists will probably never be certain of the largest and smallest dinosaurs.

The tallest and heaviest dinosaur known from good skeletons is Giraffatitan brancai (previously classified as a species of Brachiosaurus). Its remains were discovered in Tanzania between 1907–12. Bones from multiple similar-sized individuals were incorporated into the skeleton now mounted and on display at the Humboldt Museum of Berlin;[52] this mount is 12 meters (39 ft) tall and 22.5 meters (74 ft) long, and would have belonged to an animal that weighed between 30000 and 60000 kilograms (70000 and 130000 lb). The longest complete dinosaur is the 27-meter (89 ft) long Diplodocus, which was discovered in Wyoming in the United States and displayed in Pittsburgh's Carnegie Natural History Museum in 1907.
.png)
There were larger dinosaurs, but knowledge of them is based entirely on a small number of fragmentary fossils. Most of the largest herbivorous specimens on record were all discovered in the 1970s or later, and include the massive Argentinosaurus, which may have weighed 80000 to 100000 kilograms (90 to 110 short tons); some of the longest were the 33.5 meters (110 ft) long Diplodocus hallorum[48] (formerly Seismosaurus) and the 33 meters (110 ft) long Supersaurus;[53] and the tallest, the 18 meters (59 ft) tall Sauroposeidon, which could have reached a sixth-floor window. The longest of them all may have been Amphicoelias fragillimus, known only from a now lost partial vertebral neural arch described in 1878. Extrapolating from the illustration of this bone, the animal may have been 58 meters (190 ft) long and weighed over 120000 kg (260000 lb).[48] The largest known carnivorous dinosaur was Spinosaurus, reaching a length of 16 to 18 meters (50 to 60 ft), and weighing in at 8150 kg (18000 lb).[54] Other large meat-eaters included Giganotosaurus, Carcharodontosaurus and Tyrannosaurus.[55]
Not including modern birds, the smallest dinosaurs known were about the size of a pigeon.[56] The theropods Anchiornis and Epidexipteryx both had a total skeletal length of under 35 centimeters (1.1 ft).[56][57] Anchiornis is currently the smallest dinosaur described from an adult specimen, with an estimated weight of 110 grams.[57] The smallest herbivorous dinosaurs included Microceratus and Wannanosaurus, at about 60 cm (2 ft) long each.[58][59]
Behavior

Interpretations of dinosaur behavior are generally based on the pose of body fossils and their habitat, computer simulations of their biomechanics, and comparisons with modern animals in similar ecological niches. As such, the current understanding of dinosaur behavior relies on speculation, and will likely remain controversial for the foreseeable future. However, there is general agreement that some behaviors which are common in crocodiles and birds, dinosaurs' closest living relatives, were also common among dinosaurs.
The first potential evidence of herding behavior was the 1878 discovery of 31 Iguanodon dinosaurs which were then thought to have perished together in Bernissart, Belgium, after they fell into a deep, flooded sinkhole and drowned.[60] Other mass-death sites have been subsequently discovered. Those, along with multiple trackways, suggest that gregarious behavior was common in many dinosaur species. Trackways of hundreds or even thousands of herbivores indicate that duck-bills (hadrosaurids) may have moved in great herds, like the American Bison or the African Springbok. Sauropod tracks document that these animals traveled in groups composed of several different species, at least in Oxfordshire, England,[61] although there is not evidence for specific herd structures.[62] Dinosaurs may have congregated in herds for defense, for migratory purposes, or to provide protection for their young. There is evidence that many types of dinosaurs, including various theropods, sauropods, ankylosaurians, ornithopods, and ceratopsians, formed aggregations of immature individuals. One example is a site in Inner Mongolia that has yielded the remains of over twenty Sinornithomimus, from one to seven years old. This assemblage is interpreted as a social group that was trapped in mud.[63] The interpretation of dinosaurs as gregarious has also extended to depicting carnivorous theropods as pack hunters working together to bring down large prey.[64][65] However, this lifestyle is uncommon among the modern relatives of dinosaurs (crocodiles and other reptiles, and birds – Harris's Hawk is a well-documented exception), and the taphonomic evidence suggesting pack hunting in such theropods as Deinonychus and Allosaurus can also be interpreted as the results of fatal disputes between feeding animals, as is seen in many modern diapsid predators.[66]
Jack Horner's 1978 discovery of a Maiasaura ("good mother dinosaur") nesting ground in Montana demonstrated that parental care continued long after birth among the ornithopods.[67] There is also evidence that other Cretaceous-era dinosaurs, like Patagonian titanosaurian sauropods (1997 discovery), also nested in large groups.[68] The Mongolian oviraptorid Citipati was discovered in a chicken-like brooding position in 1993, which may mean it was covered with an insulating layer of feathers that kept the eggs warm.[69] Parental care is also implied by other finds. For example, the fossilized remains of a grouping of Psittacosaurus has been found, consisting of one adult and 34 juveniles; in this case, the large number of juveniles may be due to communal nesting.[70] Additionally, a dinosaur embryo (pertaining to the prosauropod Massospondylus) was found without teeth, indicating that some parental care was required to feed the young dinosaur.[71] Trackways have also confirmed parental behavior among ornithopods from the Isle of Skye in northwestern Scotland.[72] Nests and eggs have been found for most major groups of dinosaurs, and it appears likely that dinosaurs communicated with their young, in a manner similar to modern birds and crocodiles.

The crests and frills of some dinosaurs, like the marginocephalians, theropods and lambeosaurines, may have been too fragile to be used for active defense, and so they were likely used for sexual or aggressive displays, though little is known about dinosaur mating and territorialism. Head wounds from bites suggest that theropods, at least, engaged in active aggressive confrontations.[73] The nature of dinosaur communication also remains enigmatic, and is an active area of research. For example, recent studies suggest that the hollow crests of the lambeosaurines may have functioned as resonance chambers used for a wide range of vocalizations.[74][75]
From a behavioral standpoint, one of the most valuable dinosaur fossils was discovered in the Gobi Desert in 1971. It included a Velociraptor attacking a Protoceratops,[76] providing evidence that dinosaurs did indeed attack each other.[77] Additional evidence for attacking live prey is the partially healed tail of an Edmontosaurus, a hadrosaurid dinosaur; the tail is damaged in such a way that shows the animal was bitten by a tyrannosaur but survived.[77] Cannibalism amongst some species of dinosaurs was confirmed by tooth marks found in Madagascar in 2003, involving the theropod Majungasaurus.[78]
Based on current fossil evidence from dinosaurs such as Oryctodromeus, some herbivorous species seem to have led a partially fossorial (burrowing) lifestyle,[79] and some bird-like species may have been arboreal (tree-climbing), most notably primitive dromaeosaurids such as Microraptor[80] and the enigmatic scansoriopterygids.[81] However, most dinosaurs seem to have relied on land-based locomotion. A good understanding of how dinosaurs moved on the ground is key to models of dinosaur behavior; the science of biomechanics, in particular, has provided significant insight in this area. For example, studies of the forces exerted by muscles and gravity on dinosaurs' skeletal structure have investigated how fast dinosaurs could run,[82] whether diplodocids could create sonic booms via whip-like tail snapping,[83] and whether sauropods could float.[84]
Physiology

A vigorous debate on the subject of temperature regulation in dinosaurs has been ongoing since the 1960s. Originally, scientists broadly disagreed as to whether dinosaurs were capable of regulating their body temperatures at all. More recently, dinosaur endothermy has become the consensus view, and debate has focused on the mechanisms of temperature regulation.
After dinosaurs were discovered, paleontologists first posited that they were ectothermic creatures: "terrible lizards" as their name suggests. This supposed cold-bloodedness implied that dinosaurs were relatively slow, sluggish organisms, comparable to modern reptiles, which need external sources of heat in order to regulate their body temperature. Dinosaur ectothermy remained a prevalent view until Robert T. "Bob" Bakker, an early proponent of dinosaur endothermy, published an influential paper on the topic in 1968.
Modern evidence indicates that dinosaurs thrived in cooler temperate climates, and that at least some dinosaur species must have regulated their body temperature by internal biological means (perhaps aided by the animals' bulk). Evidence of endothermy in dinosaurs includes the discovery of polar dinosaurs in Australia and Antarctica (where they would have experienced a cold, dark six-month winter), the discovery of dinosaurs whose feathers may have provided regulatory insulation, and analysis of blood-vessel structures within dinosaur bone that are typical of endotherms. Skeletal structures suggest that theropods and other dinosaurs had active lifestyles better suited to an endothermic cardiovascular system, while sauropods exhibit fewer endothermic characteristics. It is certainly possible that some dinosaurs were endothermic while others were not. Scientific debate over the specifics continues.[85]
Complicating the debate is the fact that warm-bloodedness can emerge based on more than one mechanism. Most discussions of dinosaur endothermy tend to compare them with average-sized birds or mammals, which expend energy to elevate body temperature above that of the environment. Small birds and mammals also possess insulation, such as fat, fur, or feathers, which slows down heat loss. However, large mammals, such as elephants, face a different problem because of their relatively small ratio of surface area to volume (Haldane's principle). This ratio compares the volume of an animal with the area of its skin: as an animal gets bigger, its surface area increases more slowly than its volume. At a certain point, the amount of heat radiated away through the skin drops below the amount of heat produced inside the body, forcing animals to use additional methods to avoid overheating. In the case of elephants, they have little hair as adults, have large ears which increase their surface area, and have behavioral adaptations as well (such as using the trunk to spray water on themselves and mud-wallowing). These behaviors increase cooling through evaporation.
Large dinosaurs would presumably have had to deal with similar issues; their body size suggest they lost heat relatively slowly to the surrounding air, and so could have been what are called inertial homeotherms, animals that are warmer than their environments through sheer size rather than through special adaptations like those of birds or mammals. However, so far this theory fails to account for the numerous dog- and goat-sized dinosaur species, or the young of larger species.
Modern computerized tomography (CT) scans of a dinosaur's chest cavity (conducted in 2000) found the apparent remnants of a four-chambered heart, much like those found in today's mammals and birds.[86] The idea is controversial within the scientific community, coming under fire for bad anatomical science[87] or simply wishful thinking.[88] The question of how this find reflects on metabolic rate and dinosaur internal anatomy may be moot, though, regardless of the object's identity: both modern crocodilians and birds, the closest living relatives of dinosaurs, have four-chambered hearts (albeit modified in crocodilians), and so dinosaurs probably had them as well.[89]
Soft tissue and DNA
One of the best examples of soft-tissue impressions in a fossil dinosaur was discovered in Petraroia, Italy. The discovery was reported in 1998, and described the specimen of a small, very young coelurosaur, Scipionyx samniticus. The fossil includes portions of the intestines, colon, liver, muscles, and windpipe of this immature dinosaur.[45]
In the March 2005 issue of Science, the paleontologist Mary Higby Schweitzer and her team announced the discovery of flexible material resembling actual soft tissue inside a 68-million-year-old Tyrannosaurus rex leg bone from the Hell Creek Formation in Montana. After recovery, the tissue was rehydrated by the science team.[46]
When the fossilized bone was treated over several weeks to remove mineral content from the fossilized bone-marrow cavity (a process called demineralization), Schweitzer found evidence of intact structures such as blood vessels, bone matrix, and connective tissue (bone fibers). Scrutiny under the microscope further revealed that the putative dinosaur soft tissue had retained fine structures (microstructures) even at the cellular level. The exact nature and composition of this material, and the implications of Schweitzer's discovery, are not yet clear; study and interpretation of the material is ongoing.[46]
Newer research, published in PloS One (30 July 2008), has challenged the claims that the material found is the soft tissue of Tyrannosaurus. Thomas Kaye of the University of Washington and his co-authors contend that what was really inside the tyrannosaur bone was slimy biofilm created by bacteria that coated the voids once occupied by blood vessels and cells.[90] The researchers found that what previously had been identified as remnants of blood cells, because of the presence of iron, were actually framboids, microscopic mineral spheres bearing iron. They found similar spheres in a variety of other fossils from various periods, including an ammonite. In the ammonite they found the spheres in a place where the iron they contain could not have had any relationship to the presence of blood.[91]
The successful extraction of ancient DNA from dinosaur fossils has been reported on two separate occasions, but, upon further inspection and peer review, neither of these reports could be confirmed.[92] However, a functional visual peptide of a theoretical dinosaur has been inferred using analytical phylogenetic reconstruction methods on gene sequences of related modern species such as reptiles and birds.[93] In addition, several proteins, including hemoglobin,[94] have putatively been detected in dinosaur fossils.[95]
Feathers and the origin of birds
The possibility that dinosaurs were the ancestors of birds was first suggested in 1868 by Thomas Henry Huxley.[96] After the work of Gerhard Heilmann in the early 20th century, the theory of birds as dinosaur descendants was abandoned in favor of the idea of their being descendants of generalized thecodonts, with the key piece of evidence being the supposed lack of clavicles in dinosaurs.[97] However, as later discoveries showed, clavicles (or a single fused wishbone, which derived from separate clavicles) were not actually absent;[13] they had been found as early as 1924 in Oviraptor, but misidentified as an interclavicle.[98] In the 1970s, John Ostrom revived the dinosaur–bird theory,[99] which gained momentum in the coming decades with the advent of cladistic analysis,[100] and a great increase in the discovery of small theropods and early birds.[20] Of particular note have been the fossils of the Yixian Formation, where a variety of theropods and early birds have been found, often with feathers of some type.[13] Birds share over a hundred distinct anatomical features with theropod dinosaurs, which are now generally accepted to have been their closest ancient relatives.[101] They are most closely allied with maniraptoran coelurosaurs.[13] A minority of scientists, most notably Alan Feduccia and Larry Martin, have proposed other evolutionary paths, including revised versions of Heilmann's basal archosaur proposal,[102] or that maniraptoran theropods are the ancestors of birds but themselves are not dinosaurs, only convergent with dinosaurs.[103]
Feathers

Archaeopteryx, the first good example of a "feathered dinosaur", was discovered in 1861. The initial specimen was found in the Solnhofen limestone in southern Germany, which is a lagerstätte, a rare and remarkable geological formation known for its superbly detailed fossils. Archaeopteryx is a transitional fossil, with features clearly intermediate between those of modern reptiles and birds. Brought to light just two years after Darwin's seminal The Origin of Species, its discovery spurred the nascent debate between proponents of evolutionary biology and creationism. This early bird is so dinosaur-like that, without a clear impression of feathers in the surrounding rock, at least one specimen was mistaken for Compsognathus.[104]
Since the 1990s, a number of additional feathered dinosaurs have been found, providing even stronger evidence of the close relationship between dinosaurs and modern birds. Most of these specimens were unearthed in the lagerstätte of the Yixian Formation, Liaoning, northeastern China, which was part of an island continent during the Cretaceous. Though feathers have been found in only a few locations, it is possible that non-avian dinosaurs elsewhere in the world were also feathered. The lack of widespread fossil evidence for feathered non-avian dinosaurs may be because delicate features like skin and feathers are not often preserved by fossilization and thus are absent from the fossil record. To this point, protofeathers (thin, filament-like structures) are known from dinosaurs at the base of Coelurosauria, such as compsognathids like Sinosauropteryx and tyrannosauroids (Dilong),[105] but barbed feathers are known only among the coelurosaur subgroup Maniraptora, which includes oviraptorosaurs, troodontids, dromaeosaurids, and birds.[13][106] The description of feathered dinosaurs has not been without controversy; perhaps the most vocal critics have been Alan Feduccia and Theagarten Lingham-Soliar, who have proposed that protofeathers are the result of the decomposition of collagenous fiber that underlaid the dinosaurs' integument,[107][108][109] and that maniraptoran dinosaurs with barbed feathers were not actually dinosaurs, but convergent with dinosaurs.[103][108] However, their views have for the most part not been accepted by other researchers, to the point that the question of the scientific nature of Feduccia's proposals has been raised.[110]
Skeleton
Because feathers are often associated with birds, feathered dinosaurs are often touted as the missing link between birds and dinosaurs. However, the multiple skeletal features also shared by the two groups represent another important line of evidence for paleontologists. Areas of the skeleton with important similarities include the neck, pubis, wrist (semi-lunate carpal), arm and pectoral girdle, furcula (wishbone), and breast bone. Comparison of bird and dinosaur skeletons through cladistic analysis strengthens the case for the link.
Soft anatomy

Large meat-eating dinosaurs had a complex system of air sacs similar to those found in modern birds, according to an investigation which was led by Patrick O'Connor of Ohio University. The lungs of theropod dinosaurs (carnivores that walked on two legs and had bird-like feet) likely pumped air into hollow sacs in their skeletons, as is the case in birds. "What was once formally considered unique to birds was present in some form in the ancestors of birds", O'Connor said.[111] In a 2008 paper published in the online journal PLoS ONE, scientists described Aerosteon riocoloradensis, the skeleton of which supplies the strongest evidence to date of a dinosaur with a bird-like breathing system. CT-scanning revealed the evidence of air sacs within the body cavity of the Aerosteon skeleton.[112][113]
Another piece of evidence that birds and dinosaurs are closely related is the use by both of gizzard stones. These stones are swallowed by animals to aid digestion and break down food and hard fibers once they enter the stomach. When found in association with fossils, gizzard stones are called gastroliths.[114]
Reproductive biology
A discovery of features in a Tyrannosaurus rex skeleton recently provided more evidence that dinosaurs and birds evolved from a common ancestor and, for the first time, allowed paleontologists to establish the sex of a dinosaur. When laying eggs, female birds grow a special type of bone between the hard outer bone and the marrow of their limbs. This medullary bone, which is rich in calcium, is used to make eggshells. The presence of endosteally derived bone tissues lining the interior marrow cavities of portions of the Tyrannosaurus rex specimen's hind limb suggested that T. rex used similar reproductive strategies, and revealed the specimen to be female.[115] Further research has found medullary bone in the theropod Allosaurus and the ornithopod Tenontosaurus. Because the line of dinosaurs that includes Allosaurus and Tyrannosaurus diverged from the line that led to Tenontosaurus very early in the evolution of dinosaurs, this suggests that dinosaurs in general produced medullary tissue. Medullary bone has been found in specimens of sub-adult size, which suggests that dinosaurs reached sexual maturity rather quickly for such large animals.[116]
Behavioral evidence
A recently discovered troodont fossil demonstrates that some dinosaurs slept with their heads tucked under their arms.[117] This behavior, which may have helped to keep the head warm, is also characteristic of modern birds.
Extinction
Non-avian dinosaurs suddenly became extinct approximately 65 million years ago. Many other groups of animals also became extinct at this time, including ammonites (nautilus-like mollusks), mosasaurs, plesiosaurs, pterosaurs, most birds, and many groups of mammals.[5] This mass extinction is known as the Cretaceous–Tertiary extinction event. The nature of the event that caused this mass extinction has been extensively studied since the 1970s; at present, several related theories are supported by paleontologists. Though the consensus is that an impact event was the primary cause of dinosaur extinction, some scientists cite other possible causes, or support the idea that a confluence of several factors was responsible for the sudden disappearance of dinosaurs from the fossil record.
At the peak of the Mesozoic, there were no polar ice caps, and sea levels are estimated to have been from 100 to 250 meters (300 to 800 ft) higher than they are today. The planet's temperature was also much more uniform, with only 25 °C (45 °F) separating average polar temperatures from those at the equator. On average, atmospheric temperatures were also much higher; the poles, for example, were 50 °C (90 °F) warmer than today.[118][119]
The atmosphere's composition during the Mesozoic was vastly different as well. Carbon dioxide levels were up to 12 times higher than today's levels, and oxygen formed 32 to 35% of the atmosphere, as compared to 21% today. However, by the late Cretaceous, the environment was changing dramatically. Volcanic activity was decreasing, which led to a cooling trend as levels of atmospheric carbon dioxide dropped. Oxygen levels in the atmosphere also started to fluctuate and would ultimately fall considerably. Some scientists hypothesize that climate change, combined with lower oxygen levels, might have led directly to the demise of many species. If the dinosaurs had respiratory systems similar to those commonly found in modern birds, it may have been particularly difficult for them to cope with reduced respiratory efficiency, given the enormous oxygen demands of their very large bodies.[5]
Impact event

The asteroid collision theory, which was brought to wide attention in 1980 by Walter Alvarez and colleagues, links the extinction event at the end of the Cretaceous period to a bolide impact approximately 65.5 million years ago. Alvarez et al. proposed that a sudden increase in iridium levels, recorded around the world in the period's rock stratum, was direct evidence of the impact.[120] The bulk of the evidence now suggests that a 5 to 15 kilometers (3 to 9 mi) wide bolide hit in the vicinity of the Yucatán Peninsula, creating the approximately 180 kilometers (110 mi) Chicxulub Crater and triggering the mass extinction.[121][122] Scientists are not certain whether dinosaurs were thriving or declining before the impact event. Some scientists propose that the meteorite caused a long and unnatural drop in Earth's atmospheric temperature, while others claim that it would have instead created an unusual heat wave.
Although the speed of extinction cannot be deduced from the fossil record alone, various models suggest that the extinction was extremely rapid. The consensus among scientists who support this theory is that the impact caused extinctions both directly (by heat from the meteorite impact) and also indirectly (via a worldwide cooling brought about when matter ejected from the impact crater reflected thermal radiation from the sun).
In September 2007, U.S. researchers led by William Bottke of the Southwest Research Institute in Boulder, Colorado, and Czech scientists used computer simulations to identify the probable source of the Chicxulub impact. They calculated a 90% probability that a giant asteroid named Baptistina, approximately 160 kilometers (100 mi) in diameter, orbiting in the asteroid belt which lies between Mars and Jupiter, was struck by a smaller unnamed asteroid about 55 kilometers (35 mi) in diameter about 160 million years ago. The impact shattered Baptistina, creating a cluster which still exists today as the Baptistina family. Calculations indicate that some of the fragments were sent hurtling into earth-crossing orbits, one of which was the 10 kilometers (6 mi) wide meteorite which struck Mexico's Yucatan peninsula 65 million years ago, creating the Chicxulub crater.[123]
A similar but more controversial explanation proposes that "passages of the [hypothetical] solar companion star Nemesis through the Oort comet cloud would trigger comet showers."[124] One or more of these comets then collided with the Earth at approximately the same time, causing the worldwide extinction. As with the impact of a single asteroid, the end result of this comet bombardment would have been a sudden drop in global temperatures, followed by a protracted cool period.[124]
Deccan Traps
Before 2000, arguments that the Deccan Traps flood basalts caused the extinction were usually linked to the view that the extinction was gradual, as the flood basalt events were thought to have started around 68 million years ago and lasted for over 2 million years. However, there is evidence that two-thirds of the Deccan Traps were created in only 1 million years about 65.5 million years ago, and so these eruptions would have caused a fairly rapid extinction, possibly over a period of thousands of years, but still longer than would be expected from a single impact event.[125][126]
The Deccan Traps could have caused extinction through several mechanisms, including the release into the air of dust and sulphuric aerosols, which might have blocked sunlight and thereby reduced photosynthesis in plants. In addition, Deccan Trap volcanism might have resulted in carbon dioxide emissions, which would have increased the greenhouse effect when the dust and aerosols cleared from the atmosphere.[126] Before the mass extinction of the dinosaurs, the release of volcanic gases during the formation of the Deccan Traps "contributed to an apparently massive global warming. Some data point to an average rise in temperature of 8 °C (14 °F) in the last half million years before the impact [at Chicxulub]."[125][126]
In the years when the Deccan Traps theory was linked to a slower extinction, Luis Alvarez (who died in 1988) replied that paleontologists were being misled by sparse data. While his assertion was not initially well-received, later intensive field studies of fossil beds lent weight to his claim. Eventually, most paleontologists began to accept the idea that the mass extinctions at the end of the Cretaceous were largely or at least partly due to a massive Earth impact. However, even Walter Alvarez has acknowledged that there were other major changes on Earth even before the impact, such as a drop in sea level and massive volcanic eruptions that produced the Indian Deccan Traps, and these may have contributed to the extinctions.[127]
Failure to adapt to changing conditions
Lloyd et al. (2008) noted that, in the Mid Cretaceous, the flowering, angiosperm plants became a major part of terrestrial ecosystems, which had previously been dominated by gymnosperms such as conifers. Dinosaur coprolites – fossilized dung – indicate that, while some ate angiosperms, most herbivorous dinosaurs ate mainly gymnosperms. Statistical analysis by Lloyd et al. concluded that, contrary to earlier studies, dinosaurs did not diversify very much in the Late Cretaceous. Lloyd et al. suggested that dinosaurs' failure to diversify as ecosystems were changing doomed them to extinction.[36]
Possible Paleocene survivors
Non-avian dinosaur remains are occasionally found above the K-T boundary. In 2001, paleontologists Zielinski and Budahn reported the discovery of a single hadrosaur leg-bone fossil in the San Juan Basin, New Mexico, and described it as evidence of Paleocene dinosaurs. The formation in which the bone was discovered has been dated to the early Paleocene epoch, approximately 64.5 million years ago. If the bone was not re-deposited into that stratum by weathering action, it would provide evidence that some dinosaur populations may have survived at least a half million years into the Cenozoic Era.[128] Other evidence includes the finding of dinosaur remains in the Hell Creek Formation up to 1.3 meters (51 in) above (40000 years later than) the K-T boundary. Similar reports have come from other parts of the world, including China.[129] Many scientists, however, dismiss the "Paleocene dinosaurs" as re-worked, i.e. washed out of their original locations and then re-buried in much later sediments,[130][131] or find that, if correct, the presence of a handful of dinosaurs in the early Paleocene would not change the underlying facts of the extinction.[130]
History of discovery
Dinosaur fossils have been known for millennia, although their true nature was not recognized. The Chinese, whose modern word for dinosaur is konglong (恐龍, or "terrible dragon"), considered them to be dragon bones and documented them as such. For example, Hua Yang Guo Zhi, a book written by Zhang Qu during the Western Jin Dynasty, reported the discovery of dragon bones at Wucheng in Sichuan Province.[132] Villagers in central China have long unearthed fossilized "dragon bones" for use in traditional medicines, a practice that continues today.[133] In Europe, dinosaur fossils were generally believed to be the remains of giants and other creatures killed by the Great Flood.
Scholarly descriptions of what would now be recognized as dinosaur bones first appeared in the late 17th century in England. Part of a bone, now known to have been the femur of a Megalosaurus,[134] was recovered from a limestone quarry at Cornwell near Chipping Norton, Oxfordshire, England, in 1676. The fragment was sent to Robert Plot, Professor of Chemistry at the University of Oxford and first curator of the Ashmolean Museum, who published a description in his Natural History of Oxfordshire in 1677. He correctly identified the bone as the lower extremity of the femur of a large animal, and recognized that it was too large to belong to any known species. He therefore concluded it to be the thigh bone of a giant human similar to those mentioned in the Bible. In 1699, Edward Lhuyd, a friend of Sir Isaac Newton, was responsible for the first published scientific treatment of what would now be recognized as a dinosaur when he described and named a sauropod tooth, "Rutellum implicatum"[135][136], that had been found in Caswell, near Witney, Oxfordshire.[137]

Between 1815 and 1824, the Rev William Buckland, a professor of geology at Oxford University, collected more fossilized bones of Megalosaurus and became the first person to describe a dinosaur in a scientific journal.[134][138] The second dinosaur genus to be identified, Iguanodon, was discovered in 1822 by Mary Ann Mantell – the wife of English geologist Gideon Mantell. Gideon Mantell recognized similarities between his fossils and the bones of modern iguanas. He published his findings in 1825.[139][140]
The study of these "great fossil lizards" soon became of great interest to European and American scientists, and in 1842 the English paleontologist Richard Owen coined the term "dinosaur". He recognized that the remains that had been found so far, Iguanodon, Megalosaurus and Hylaeosaurus, shared a number of distinctive features, and so decided to present them as a distinct taxonomic group. With the backing of Prince Albert of Saxe-Coburg-Gotha, the husband of Queen Victoria, Owen established the Natural History Museum in South Kensington, London, to display the national collection of dinosaur fossils and other biological and geological exhibits.
In 1858, the first known American dinosaur was discovered, in marl pits in the small town of Haddonfield, New Jersey (although fossils had been found before, their nature had not been correctly discerned). The creature was named Hadrosaurus foulkii. It was an extremely important find: Hadrosaurus was one of the first nearly complete dinosaur skeletons found (the first was in 1834, in Maidstone, Kent, England), and it was clearly a bipedal creature. This was a revolutionary discovery as, until that point, most scientists had believed dinosaurs walked on four feet, like other lizards. Foulke's discoveries sparked a wave of dinosaur mania in the United States.


Dinosaur mania was exemplified by the fierce rivalry between Edward Drinker Cope and Othniel Charles Marsh, both of whom raced to be the first to find new dinosaurs in what came to be known as the Bone Wars. The feud probably originated when Marsh publicly pointed out that Cope's reconstruction of an Elasmosaurus skeleton was flawed: Cope had inadvertently placed the plesiosaur's head at what should have been the animal's tail end. The fight between the two scientists lasted for over 30 years, ending in 1897 when Cope died after spending his entire fortune on the dinosaur hunt. Marsh 'won' the contest primarily because he was better funded through a relationship with the US Geological Survey. Unfortunately, many valuable dinosaur specimens were damaged or destroyed due to the pair's rough methods: for example, their diggers often used dynamite to unearth bones (a method modern paleontologists would find appalling). Despite their unrefined methods, the contributions of Cope and Marsh to paleontology were vast: Marsh unearthed 86 new species of dinosaur and Cope discovered 56, a total of 142 new species. Cope's collection is now at the American Museum of Natural History in New York, while Marsh's is on display at the Peabody Museum of Natural History at Yale University.[141]
Since 1897, the search for dinosaur fossils has extended to every continent, including Antarctica. The first Antarctic dinosaur to be discovered, the ankylosaurid Antarctopelta oliveroi, was found on Ross Island in 1986, although it was 1994 before an Antarctic species, the theropod Cryolophosaurus ellioti, was formally named and described in a scientific journal.
Current dinosaur "hot spots" include southern South America (especially Argentina) and China. China in particular has produced many exceptional feathered dinosaur specimens due to the unique geology of its dinosaur beds, as well as an ancient arid climate particularly conducive to fossilization.
The "dinosaur renaissance"
The field of dinosaur research has enjoyed a surge in activity that began in the 1970s and is ongoing. This was triggered, in part, by John Ostrom's discovery of Deinonychus, an active predator that may have been warm-blooded, in marked contrast to the then-prevailing image of dinosaurs as sluggish and cold-blooded. Vertebrate paleontology has become a global science. Major new dinosaur discoveries have been made by paleontologists working in previously unexploited regions, including India, South America, Madagascar, Antarctica, and most significantly China (the amazingly well-preserved feathered dinosaurs in China have further consolidated the link between dinosaurs and their conjectured living descendants, modern birds). The widespread application of cladistics, which rigorously analyzes the relationships between biological organisms, has also proved tremendously useful in classifying dinosaurs. Cladistic analysis, among other modern techniques, helps to compensate for an often incomplete and fragmentary fossil record.
Cultural depictions
By human standards, dinosaurs were creatures of fantastic appearance and often enormous size. As such, they have captured the popular imagination and become an enduring part of human culture. Entry of the word "dinosaur" into the common vernacular reflects the animals' cultural importance: in English, "dinosaur" is commonly used to describe anything that is impractically large, slow-moving, obsolete, or bound for extinction.[6]
Public enthusiasm for dinosaurs first developed in Victorian England, where in 1854, three decades after the first scientific descriptions of dinosaur remains, the famous dinosaur sculptures were unveiled in London's Crystal Palace Park. The Crystal Palace dinosaurs proved so popular that a strong market in smaller replicas soon developed. In subsequent decades, dinosaur exhibits opened at parks and museums around the world, ensuring that successive generations would be introduced to the animals in an immersive and exciting way.[142] Dinosaurs' enduring popularity, in its turn, has resulted in significant public funding for dinosaur science, and has frequently spurred new discoveries. In the United States, for example, the competition between museums for public attention led directly to the Bone Wars of the 1880s and 1890s, during which a pair of feuding paleontologists made enormous scientific contributions.[143]
The popular preoccupation with dinosaurs has ensured their appearance in literature, film and other media. Beginning in 1852 with a passing mention in Charles Dickens' Bleak House,[144] dinosaurs have been featured in large numbers of fictional works. Sir Arthur Conan Doyle's 1912 book The Lost World, the iconic 1933 film King Kong, 1954's Godzilla and its many sequels, the best-selling 1990 novel Jurassic Park by Michael Crichton and its 1993 film adaptation are just a few notable examples of dinosaur appearances in fiction. Authors of general-interest non-fictional works about dinosaurs, including some prominent paleontologists, have often sought to use the animals as a way to educate readers about science in general. Dinosaurs are ubiquitous in advertising; numerous companies have referenced dinosaurs in printed or televised advertisements, either in order to sell their own products or in order to characterize their rivals as slow-moving, dim-witted or obsolete.[145]
See also
- Evolutionary history of life
- List of dinosaurs
- List of dinosaur-bearing rock formations
- Physiology of dinosaurs
- Prehistoric reptile
Notes and references
- ↑ Gauthier, Jacques; de Querioz, Kevin (2001). "Feathered dinosaurs, flying dinosaurs, crown dinosaurs, and the name 'Aves'." (PDF). New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom. Peabody Museum of Natural History, Yale University. ISBN 0-912532-57-2. http://vertebrates.si.edu/herps/herps_pdfs/deQueiroz_pdfs/2001gaudeqost.pdf. Retrieved 2009-09-22.
- ↑ Alfaro, M.E., F. Santini, C. Brock, H. Alamillo, A. Dornburg. D.L. Rabosky, G. Carnevale, and L.J. Harmon (2009). "Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates". Proceedings of the National Academy of Sciences USA 106 (32): 13410–13414. doi:10.1073/pnas.0811087106.
- ↑ 3.0 3.1 Wang, S.C., and Dodson, P. (2006). "Estimating the Diversity of Dinosaurs". Proceedings of the National Academy of Sciences USA 103 (37): 13601–13605. doi:10.1073/pnas.0606028103. PMID 16954187.
- ↑ 4.0 4.1 Will the real dinosaurs stand up?, BBC, September 17, 2008
- ↑ 5.0 5.1 5.2 5.3 MacLeod, N, Rawson, PF, Forey, PL, Banner, FT, Boudagher-Fadel, MK, Bown, PR, Burnett, JA, Chambers, P, Culver, S, Evans, SE, Jeffery, C, Kaminski, MA, Lord, AR, Milner, AC, Milner, AR, Morris, N, Owen, E, Rosen, BR, Smith, AB, Taylor, PD, Urquhart, E & Young, JR (1997). "The Cretaceous–Tertiary biotic transition". Journal of the Geological Society 154 (2): 265–292. doi:10.1144/gsjgs.154.2.0265. http://findarticles.com/p/articles/mi_qa3721/is_199703/ai_n8738406/print.
- ↑ 6.0 6.1 "Definition of dinosaur" Merriam-Webster's Online Dictionary. Accessed 26 May 2007.
- ↑ 7.0 7.1 Owen, R. (1842). "Report on British Fossil Reptiles." Part II. Report of the Eleventh Meeting of the British Association for the Advancement of Science; Held at Plymouth in July 1841. London: John Murray, Albemarle Street, 1842. Pages 60–204.
- ↑ "Liddell-Scott-Jones Lexicon of Classical Greek". http://www.perseus.tufts.edu/cgi-bin/lexindex?lookup=deino/s&lang=greek&doc=Perseus:text:1999.01.0169&formentry=0. Retrieved 2008-08-05.
- ↑ Farlow, J.O., and Brett-Surman, M.K. (1997). "Preface". In Farlow, J.O., and Brett-Surman, M.K. (eds.). The Complete Dinosaur. Indiana University Press. pp. ix-xi. ISBN 0-253-33349-0.
- ↑ "dinosaur – Definition from the Merriam-Webster Online Dictionary". http://www.merriam-webster.com/dictionary/dinosaur. Retrieved 2008-08-05.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Benton, Michael J. (2004). "Origin and relationships of Dinosauria". In Weishampel, David B.; Dodson, Peter; and Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 7–19. ISBN 0-520-24209-2.
- ↑ Olshevsky, G. (2000). "An annotated checklist of dinosaur species by continent." Mesozoic Meanderings, 3: 1–157
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Padian, K. (2004). Basal Avialae. In: Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.). The Dinosauria (second edition). University of California Press, Berkeley, 210–231. ISBN 0-520-24209-2.
- ↑ Glut, Donald F. (1997). Dinosaurs: The Encyclopedia. Jefferson, North Carolina: McFarland & Co. pp. 40. ISBN 0-89950-917-7.
- ↑ Lambert, David; and the Diagram Group (1990). The Dinosaur Data Book. New York: Avon Books. pp. 288. ISBN 0-380-75896-3.
- ↑ Morales, Michael (1997). "Nondinosaurian vertebrates of the Mesozoic". In Farlow, James O.; and Brett-Surman, Michael K. (eds.). The Complete Dinosaur. Bloomington: Indiana University Press. pp. 607–624. ISBN 0-253-33349-0.
- ↑ Hu Yaoming; Meng, J; Wang, Y; Li, C (2005). "Large Mesozoic mammals fed on dinosaurs". Nature 433 (7022): 149–152. doi:10.1038/nature03102. PMID 15650737.
- ↑ Russell, Dale A. (1995). "China and the lost worlds of the dinosaurian era". Historical Biology 10: 3–12. doi:10.1080/10292389509380510.
- ↑ Amiot, R.; Buffetaut, E.; Lécuyer, C.; Wang, X.; Boudad, L.; Ding, Z.; Fourel, F.; Hutt, S.; Martineau, F.; Medeiros, A.; Mo, J.; Simon, L.; Suteethorn, V.; Sweetman, S.; Tong, H.; Zhang, F.; and Zhou, Z. (2010). "Oxygen isotope evidence for semi-aquatic habits among spinosaurid theropods". Geology 38 (2): 139–142. doi:10.1130/G30402.1.
- ↑ 20.0 20.1 Holtz, Jr., T.R. (2000). "Classification and evolution of the dinosaur groups". In Paul, G.S. (ed.). The Scientific American Book of Dinosaurs. St. Martin's Press. pp. 140–168. ISBN 0-312-26226-4.
- ↑ Langer, M.C., Abdala, F., Richter, M., and Benton, M.J. (1999). "A sauropodomorph dinosaur from the Upper Triassic (Carnian) of southern Brazil". Comptes Rendus de l'Academie des Sciences, Paris: Sciences de la terre et des planètes 329: 511–517.
- ↑ Nesbitt, Sterling J.; Irmis, Randall B.; Parker, William G. (2007). "A critical re-evaluation of the Late Triassic dinosaur taxa of North America". Journal of Systematic Palaeontology 5 (2): 209–243. doi:10.1017/S1477201907002040.
- ↑ This was recognized not later than 1909: "Dr. Holland and the Sprawling Sauropods". http://www.hmnh.org/library/diplodocus/holland1910.html. The arguments and many of the images are also presented in Desmond, A. (1976). Hot Blooded Dinosaurs. DoubleDay. ISBN 0385270631.
- ↑ 24.0 24.1 Benton, M.J. (2004). Vertebrate Paleontology. Blackwell Publishers. xii-452. ISBN 0-632-05614-2.
- ↑ Cowen, Richard (2004). "Dinosaurs". History of Life (4th ed.). Blackwell Publishing. pp. 151–175. ISBN 1405117567. OCLC 53970577.
- ↑ 26.0 26.1 Kubo, T.; Benton, Michael J. (2007). "Evolution of hindlimb posture in archosaurs: limb stresses in extinct vertebrates". Palaeontology 50 (6): 1519–1529. doi:10.1111/j.1475-4983.2007.00723.x.
- ↑ Seeley, H.G. (1887). "On the classification of the fossil animals commonly named Dinosauria". Proc R Soc London (Royal Society) 43: 165–171. doi:10.1098/rspl.1887.0117.
- ↑ Romer, A.S. (1956). Osteology of the Reptiles. University of Chicago. ISBN 089464985X.
- ↑ Ostrom, J.H. (1980). "The evidence of endothermy in dinosaurs". In Thomas, R.D.K. and Olson, E.C. (PDF). A cold look at the warm-blooded dinosaurs. Boulder, CO: American Association for the Advancement of Science. pp. 82–105. http://bio.fsu.edu/~amarquez/Evolutionary%20Morphology%20fall%202004/Ostrom/The%20evidence%20for%20endothermy%20in%20dinosaurs%20-%20Ostrom.pdf.
- ↑ Bakker, R. T., and Galton, P. (1974). "Dinosaur monophyly and a new class of vertebrates". Nature 248: 168–172. doi:10.1038/248168a0. http://www.nature.com/nature/journal/v248/n5444/abs/248168a0.html.
- ↑ Kump LR, Pavlov A & Arthur MA (2005). "Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia". Geology 33 (5): 397–400. doi:10.1130/G21295.1.
- ↑ Tanner LH, Lucas SG & Chapman MG (2004). "Assessing the record and causes of Late Triassic extinctions" (PDF). Earth-Science Reviews 65 (1–2): 103–139. doi:10.1016/S0012-8252(03)00082-5. http://nmnaturalhistory.org/pdf_files/TJB.pdf. Retrieved 2007-10-22.
- ↑ 33.0 33.1 Sereno PC (1999). "The evolution of dinosaurs". Science 284 (5423): 2137–2147. doi:10.1126/science.284.5423.2137. PMID 10381873.
- ↑ Sereno, P.C.; Forster, Catherine A.; Rogers, Raymond R.; Monetta, Alfredo M. (1993). "Primitive dinosaur skeleton from Argentina and the early evolution of Dinosauria". Nature 361: 64–66. doi:10.1038/361064a0.
- ↑ Brusatte, S.L.; Benton, MJ; Ruta, M; Lloyd, GT (2008). "Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs". Science 321 (5895): 1485–1488. doi:10.1126/science.1161833. PMID 18787166.
- ↑ 36.0 36.1 Lloyd, G.T., Davis, K.E., Pisani, D (22 July 2008). "Dinosaurs and the Cretaceous Terrestrial Revolution". Proceedings of the Royal Society: Biology 275 (1650): 2483. doi:10.1098/rspb.2008.0715. PMID 18647715. PMC 2603200. http://journals.royalsociety.org/content/7k63203q852h4006/. Retrieved 2008-07-28. The URL contains links to all the content, and it's all free.
- ↑ Paul, G.S. (1988). Predatory Dinosaurs of the World. New York: Simon and Schuster. pp. 248–250. ISBN 0671619462.
- ↑ Clark, J.M., Maryanska, T., and Barsbold, R. (2004). "Therizinosauroidea", in The Dinosauria, 2nd ed. 151–164.
- ↑ Norell, M.A., and Makovicky, P.J. (2004). "Dromaeosauridae", in The Dinosauria, 2nd ed. 196–210.
- ↑ 40.0 40.1 40.2 40.3 Holtz, Thomas R., Jr.; Chapman, Ralph E.; and Lamanna, Matthew C. (2004). "Mesozoic biogeography of Dinosauria". In Weishampel, David B.; Dodson, Peter; and Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 627–642. ISBN 0-520-24209-2.
- ↑ 41.0 41.1 41.2 41.3 41.4 Fastovsky, David E.; and Smith, Joshua B. (2004). "Dinosaur paleoecology". In Weishampel, David B.; Dodson, Peter; and Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 614–626. ISBN 0-520-24209-2.
- ↑ Sereno, P.C.; Wilson, JA; Witmer, LM; Whitlock, JA; Maga, A; Ide, O; Rowe, TA; Kemp, Tom (2007). "Structural extremes in a Cretaceous dinosaur". PLoS ONE 2 (11): e1230. doi:10.1371/journal.pone.0001230. PMID 18030355. PMC 2077925. http://www.plosone.org/article/fetchArticle.action?articleURI=info:doi/10.1371/journal.pone.0001230.
- ↑ Prasad, V.; Strömberg, CA; Alimohammadian, H; Sahni, A (2005). "Dinosaur coprolites and the early evolution of grasses and grazers". Science 310 (5751): 1170–1180. doi:10.1126/science.1118806. PMID 16293759.
- ↑ Archibald, J. David; and Fastovsky, David E. (2004). "Dinosaur Extinction". In Weishampel, David B.; Dodson, Peter; and Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 672–684. ISBN 0-520-24209-2.
- ↑ 45.0 45.1 Dal Sasso, C. and Signore, M. (1998)). "Exceptional soft-tissue preservation in a theropod dinosaur from Italy". Nature 292 (6674): 383–387. doi:10.1038/32884.
- ↑ 46.0 46.1 46.2 Schweitzer, M.H., Wittmeyer, J.L. and Horner, J.R. (2005). "Soft-Tissue Vessels and Cellular Preservation in Tyrannosaurus rex". Science 307 (5717): 1952–1955. doi:10.1126/science.1108397. PMID 15790853.
- ↑ "Assessment and Update Status Report on the Blue Whale Balaenoptera musculus" (PDF). Committee on the Status of Endangered Wildlife in Canada. 2002. http://www.wildwhales.org/cetaceans/blue/sr_blue_whale_e.pdf.pdf. Retrieved 2007-12-05.
- ↑ 48.0 48.1 48.2 Carpenter, Kenneth (2006). "Biggest of the big: a critical re-evaluation of the mega-sauropod Amphicoelias fragillimus". In Foster, John R.; and Lucas, Spencer G. (eds.) (pdf). Paleontology and Geology of the Upper Jurassic Morrison Formation. New Mexico Museum of Natural History and Science Bulletin 36. Albuquerque: New Mexico Museum of Natural History and Science. pp. 131–138. https://scientists.dmns.org/sites/kencarpenter/PDFs%20of%20publications/Amphicoelias.pdf.
- ↑ Farlow, James A. (1993). "On the rareness of big, fierce animals: speculations about the body sizes, population densities, and geographic ranges of predatory mammals and large, carnivorous dinosaurs". In Dodson, Peter; and Gingerich, Philip. Functional Morphology and Evolution. American Journal of Science, Special Volume 293-A. pp. 167–199.
- ↑ Peczkis, J. (1994). "Implications of body-mass estimates for dinosaurs". Journal of Vertebrate Paleontology 14(4): 520–33
- ↑ "Anatomy and evolution". National Museum of Natural History. http://paleobiology.si.edu/dinosaurs/info/everything/evo_1.html. Retrieved 2007-11-21.
- ↑ Colbert, E.H. (1968). Men and Dinosaurs: The Search in Field and Laboratory. E. P. Dutton & Company:New York, vii + 283 p. ISBN 0140212884.
- ↑ Lovelace, David M. (2007). "Morphology of a specimen of Supersaurus (Dinosauria, Sauropoda) from the Morrison Formation of Wyoming, and a re-evaluation of diplodocid phylogeny". Arquivos do Museu Nacional 65 (4): 527–544.
- ↑ dal Sasso, C., Maganuco, S., Buffetaut, E., and Mendez, M.A. (2006). New information on the skull of the enigmatic theropod Spinosaurus, with remarks on its sizes and affinities. Journal of Vertebrate Paleontology 25(4):888–896.
- ↑ Therrien, F.; and Henderson, D.M. (2007). "My theropod is bigger than yours...or not: estimating body size from skull length in theropods". Journal of Vertebrate Paleontology 27 (1): 108–115. doi:10.1671/0272-4634(2007)27[108:MTIBTY]2.0.CO;2.
- ↑ 56.0 56.1 Zhang, Fucheng; Zhou, Zhonghe; Xu, Xing; Wang, Xiaolin and Sullivan, Corwin. "A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers". <http://www.nature.com/nature/journal/v455/n7216/full/nature07447.html> Nature 455, 1105–1108 (23 October 2008) | doi:10.1038/nature07447
- ↑ 57.0 57.1 Xu, X., Zhao, Q., Norell, M., Sullivan, C., Hone, D., Erickson, G., Wang, X., Han, F. and Guo, Y. (2009). "A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin." Chinese Science Bulletin, six pages, accepted November 15, 2008.
- ↑ Holtz, Thomas R. Jr. (2008) Dinosaurs: The Most Complete, Up-to-Date Encyclopedia for Dinosaur Lovers of All Ages Supplementary Information
- ↑ Butler, R.J. and Zhao, Q. (2009). "The small-bodied ornithischian dinosaurs Micropachycephalosaurus hongtuyanensis and Wannanosaurus yansiensis from the Late Cretaceous of China." Cretaceous Research. 30(1):63–77. doi:10.1016/j.cretres.2008.03.002
- ↑ Yans J, Dejax J, Pons D, Dupuis C & Taquet P (2005). "Implications paléontologiques et géodynamiques de la datation palynologique des sédiments à faciès wealdien de Bernissart (bassin de Mons, Belgique)" (in French). Comptes Rendus Palevol 4 (1–2): 135–150. doi:10.1016/j.crpv.2004.12.003.
- ↑ Day, J.J.; Upchurch, P; Norman, DB; Gale, AS; Powell, HP (2002). "Sauropod trackways, evolution, and behavior". Science 296 (5573): 1659. doi:10.1126/science.1070167. PMID 12040187.
- ↑ Wright, Joanna L. (2005). "Steps in understanding sauropod biology". In Curry Rogers, Kristina A.; and Wilson, Jeffrey A.. The Sauropods: Evolution and Paleobiology. Berkeley: University of California Press. pp. 252–284. ISBN 0-520-24623-3.
- ↑ Varricchio, D.J. (2008). "Mud-trapped herd captures evidence of distinctive dinosaur sociality" (pdf). Acta Palaeontologica Polonica 53 (4): 567–578. doi:10.4202/app.2008.0402. http://www.app.pan.pl/article/item/app53-567.html. Retrieved 2008-12-09.
- ↑ Lessem, Don; and Glut, Donald F. (1993). "Allosaurus". The Dinosaur Society's Dinosaur Encyclopedia. Random House. pp. 19–20. ISBN 0-679-41770-2.
- ↑ Maxwell, W. D. (1995). "Taphonomy and paleobiological implications of Tenontosaurus-Deinonychus associations". Journal of Vertebrate Paleontology 15 (4): 707–712.(abstract)
- ↑ Roach, Brian T.; Brinkman, Daniel L. (2007). "A reevaluation of cooperative pack hunting and gregariousness in Deinonychus antirrhopus and other nonavian theropod dinosaurs". Bulletin of the Peabody Museum of Natural History 48 (1): 103–138. doi:10.3374/0079-032X(2007)48[103:AROCPH]2.0.CO;2.
- ↑ Horner, J.R.; Makela, Robert (1979). "Nest of juveniles provides evidence of family structure among dinosaurs". Nature 282 (5736): 296–298. doi:10.1038/282296a0.
- ↑ Chiappe, Luis M.; Jackson, Frankie; Coria, Rodolfo A.; and Dingus, Lowell (2005). "Nesting titanosaurs from Auca Mahuevo and adjacent sites". In Curry Rogers, Kristina A.; and Wilson, Jeffrey A.. The Sauropods: Evolution and Paleobiology. Berkeley: University of California Press. pp. 285–302. ISBN 0-520-24623-3.
- ↑ Oviraptor nesting Oviraptor nests or Protoceratops?
- ↑ Meng Qingjin; Liu Jinyuan; Varricchio, David J.; Huang, Timothy; and Gao Chunling (2004). "Parental care in an ornithischian dinosaur". Nature 431 (7005): 145–146. doi:10.1038/431145a. PMID 15356619.
- ↑ Reisz RR, Scott, D Sues, H-D, Evans, DC & Raath, MA (2005). "Embryos of an Early Jurassic prosauropod dinosaur and their evolutionary significance". Science 309 (5735): 761–764. doi:10.1126/science.1114942. PMID 16051793.
- ↑ Dinosaur family tracks Footprints show maternal instinct after leaving the nest.
- ↑ Tanke, Darren H. (1998). "Head-biting behavior in theropod dinosaurs: paleopathological evidence" (pdf). Gaia (15): 167–184. ISSN 0871-5424. http://www.mnhn.ul.pt/geologia/gaia/12.pdf.
- ↑ Hopson, James A. (1975). "The evolution of cranial display structures in hadrosaurian dinosaurs". Paleobiology 1 (1): 21–43.
- ↑ Diegert, Carl F. (1998). "A digital acoustic model of the lambeosaurine hadrosaur Parasaurolophus tubicen". Journal of Vertebrate Paleontology 18 (3, Suppl.): 38A.
- ↑ "The Fighting Dinosaurs". American Museum of Natural History. http://www.amnh.org/exhibitions/fightingdinos/ex-fd.html. Retrieved 2007-12-05.
- ↑ 77.0 77.1 Carpenter, K. (1998). "Evidence of predatory behavior by theropod dinosaurs". Gaia 15: 135–144. http://vertpaleo.org/publications/jvp/15-576-591.cfm. Retrieved 2007-12-05.
- ↑ Rogers, Raymond R.; Krause, DW; Curry Rogers, K (2007). "Cannibalism in the Madagascan dinosaur Majungatholus atopus". Nature 422 (6931): 515–518. doi:10.1038/nature01532. PMID 12673249.
- ↑ Varricchio DJ, Martin, AJ and Katsura, Y (2007). "First trace and body fossil evidence of a burrowing, denning dinosaur". Proceedings of the Royal Society B: Biological Sciences 274 (1616): 1361–1368. doi:10.1098/rspb.2006.0443. PMID 17374596.
- ↑ Chatterjee, S.; Templin, R. J. (2007). "Biplane wing planform and flight performance of the feathered dinosaur Microraptor gui" (pdf). Proceedings of the National Academy of Sciences 104 (5): 1576–1580. doi:10.1073/pnas.0609975104. PMID 17242354. PMC 1780066. http://www.pnas.org/cgi/reprint/0609975104v1.pdf.
- ↑ Zhang, F.; Zhou, Z.; Xu, X.; and Wang, X. (2002). "A juvenile coelurosaurian theropod from China indicates arboreal habits". Naturwissenschaften 89 (9): 394–398. doi:10.1007/s00114-002-0353-8. PMID 12435090.
- ↑ Alexander RM (2006). "Dinosaur biomechanics". Proceedings of the Royal Society of Biological Sciences 273 (1596): 1849–1855. doi:10.1098/rspb.2006.3532. PMID 16822743.
- ↑ Goriely A & McMillen T (2002). "Shape of a cracking whip". Physical Review Letters 88 (24): 244301. doi:10.1103/PhysRevLett.88.244301. PMID 12059302.
- ↑ Henderson, D.M. (2003). "Effects of stomach stones on the buoyancy and equilibrium of a floating crocodilian: A computational analysis". Canadian Journal of Zoology 81 (8): 1346–1357. doi:10.1139/z03-122.
- ↑ Parsons, K.M. (2001). Drawing Out Leviathan. Indiana University Press. 22–48. ISBN 0-253-33937-5.
- ↑ Fisher, P. E., Russell, D. A., Stoskopf, M. K., Barrick, R. E., Hammer, M. & Kuzmitz, A. A. (2000). "Cardiovascular evidence for an intermediate or higher metabolic rate in an ornithischian dinosaur". Science 288 (5465): 503–505. doi:10.1126/science.288.5465.503. PMID 10775107.
- ↑ Hillenius, W. J. & Ruben, J. A. (2004). "The evolution of endothermy in terrestrial vertebrates: Who? when? why?". Physiological and Biochemical Zoology 77 (6): 1019–1042. doi:10.1086/425185. PMID 15674773.
- ↑ Rowe T, McBride EF, & Sereno PC (2001). "Dinosaur with a Heart of Stone". Science 291 (5505): 783. doi:10.1126/science.291.5505.783a. PMID 11157158.
- ↑ Chinsamy, Anusuya; and Hillenius, Willem J. (2004). "Physiology of nonavian dinosaurs". The Dinosauria, 2nd. 643–659.
- ↑ Kaye, T. G.; Gaugler, G; Sawlowicz, Z; Stepanova, Anna (July 2008). "Dinosaurian Soft Tissues Interpreted as Bacterial Biofilms.". PLoS ONE 3 (7): e2808. doi:10.1371/journal.pone.0002808. PMID 18665236. PMC 2483347. http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0002808. Retrieved 2008-10-27.
- ↑ New Research Challenges Notion That Dinosaur Soft Tissues Still Survive Newswise, Retrieved on 29 July 2008.
- ↑ Wang, H., Yan, Z. and Jin, D. (1 May 1997). "Reanalysis of published DNA sequence amplified from Cretaceous dinosaur egg fossil". Molecular Biology and Evolution 14 (5): 589–591. PMID 9159936. http://mbe.oupjournals.org/cgi/reprint/14/5/589. Retrieved 2007-12-05.
- ↑ Chang BS, Jönsson K, Kazmi MA, Donoghue MJ, Sakmar TP (1 September 2002). "Recreating a Functional Ancestral Archosaur Visual Pigment". Molecular Biology and Evolution 19 (9): 1483–1489. PMID 12200476. http://mbe.oxfordjournals.org/cgi/content/full/19/9/1483. Retrieved 2007-12-05.
- ↑ Schweitzer MH, Marshall M, Carron K, Bohle DS, Busse SC, Arnold EV, Barnard D, Horner JR, Starkey JR (1997). "Heme compounds in dinosaur trabecular bone". Proc Natl Acad Sci U S A. 94 (12): 6291–6. doi:10.1073/pnas.94.12.6291. PMID 9177210.
- ↑ Embery G, Milner AC, Waddington RJ, Hall RC, Langley MS, Milan AM (2003). "Identification of proteinaceous material in the bone of the dinosaur Iguanodon". Connect Tissue Res 44 (Suppl 1): 41–6. doi:10.1080/713713598. PMID 12952172.
- ↑ Huxley, Thomas H. (1868). "On the animals which are most nearly intermediate between birds and reptiles". Annals of the Magazine of Natural History 4 (2): 66–75.
- ↑ Heilmann, Gerhard (1926). The Origin of Birds. London: Witherby. pp. 208pp. ISBN 0486227847.
- ↑ Osborn, Henry Fairfield (1924). "Three new Theropoda, Protoceratops zone, central Mongolia" (pdf). American Museum Novitates 144: 1–12. http://digitallibrary.amnh.org/dspace/bitstream/2246/3223/1/N0144.pdf.
- ↑ Ostrom, John H. (1973). "The ancestry of birds". Nature 242 (5393): 136. doi:10.1038/242136a0.
- ↑ Gauthier, Jacques. (1986). "Saurischian monophyly and the origin of birds". In Padian, Kevin. (ed.). The Origin of Birds and the Evolution of Flight. Memoirs of the California Academy of Sciences 8. pp. 1–55.
- ↑ Mayr, G., Pohl, B. and Peters, D.S. (2005). "A Well-Preserved Archaeopteryx Specimen with Theropod Features". Science 310 (5753): 1483–1486. doi:10.1126/science.1120331. PMID 16322455.
- ↑ Martin, Larry D. (2006). "A basal archosaurian origin for birds". Acta Zoologica Sinica 50 (6): 977–990.
- ↑ 103.0 103.1 Feduccia, A. (2002). "Birds are dinosaurs: simple answer to a complex problem". The Auk 119: 1187–1201. doi:10.1642/0004-8038(2002)119[1187:BADSAT]2.0.CO;2.
- ↑ Wellnhofer, P (1988). "Ein neuer Exemplar von Archaeopteryx". Archaeopteryx 6: 1–30.
- ↑ Xu X.; Norell, M.A.; Kuang X.; Wang X.; Zhao Q.; and Jia C. (2004). "Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids". Nature 431 (7009): 680–684. doi:10.1038/nature02855. PMID 15470426.
- ↑ Göhlich, U.B.; Chiappe, LM (2006). "A new carnivorous dinosaur from the Late Jurassic Solnhofen archipelago". Nature 440 (7082): 329–332. doi:10.1038/nature04579. PMID 16541071.
- ↑ Lingham-Soliar, T. (2003). "The dinosaurian origin of feathers: perspectives from dolphin (Cetacea) collagen fibers". Naturwissenschaften 12 (12): 563–567. doi:10.1007/s00114-003-0483-7. PMID 14676953.
- ↑ 108.0 108.1 Feduccia, A.; Lingham-Soliar, T; Hinchliffe, JR (2005). "Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence". Journal of Morphology 266 (2): 125–166. doi:10.1002/jmor.10382. PMID 16217748.
- ↑ Lingham-Soliar, T.; Feduccia, A; Wang, X (2007). "A new Chinese specimen indicates that 'protofeathers' in the Early Cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibres". Proceedings of the Biological Sciences 274 (1620): 1823–9. doi:10.1098/rspb.2007.0352. PMID 17521978.
- ↑ Prum, Richard O. (April 2003). "Are Current Critiques Of The Theropod Origin Of Birds Science? Rebuttal To Feduccia 2002". The Auk 120 (2): 550–61. doi:10.1642/0004-8038(2003)120[0550:ACCOTT]2.0.CO;2. http://links.jstor.org/sici?sici=0004-8038(200304)120:2%3C550:ACCOTT%3E2.0.CO;2-0.
- ↑ O'Connor, P.M. & Claessens, L.P.A.M. (2005). "Basic avian pulmonary design and flow-through ventilation in non-avian theropod dinosaurs". Nature 436 (7048): 253–256. doi:10.1038/nature03716. PMID 16015329.
- ↑ Sereno, P.C.; Martinez, RN; Wilson, JA; Varricchio, DJ; Alcober, OA; Larsson, HC; Kemp, Tom (September 2008). "Evidence for Avian Intrathoracic Air Sacs in a New Predatory Dinosaur from Argentina". PLoS ONE 3 (9): e3303. doi:10.1371/journal.pone.0003303. PMID 18825273. PMC 2553519. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0003303. Retrieved 2008-10-27.
- ↑ Meat-Eating Dinosaur from Argentina Had Bird-Like Breathing System Newswise, Retrieved on September 29, 2008.
- ↑ Wings O (2007). "A review of gastrolith function with implications for fossil vertebrates and a revised classification" (PDF). Palaeontologica Polonica 52 (1): 1–16. http://www.app.pan.pl/archive/published/app52/app52-001.pdf.
- ↑ Schweitzer, M.H.; Wittmeyer, JL; Horner, JR (2005). "Gender-specific reproductive tissue in ratites and Tyrannosaurus rex". Science 308 (5727): 1456–1460. doi:10.1126/science.1112158. PMID 15933198. http://www.sciencemag.org/cgi/content/abstract/308/5727/1456.
- ↑ Lee, Andrew H.; Werning, S (2008). "Sexual maturity in growing dinosaurs does not fit reptilian growth models". Proceedings of the National Academy of Sciences 105 (2): 582–587. doi:10.1073/pnas.0708903105. PMID 18195356. PMC 2206579. http://www.pnas.org/cgi/content/abstract/105/2/582.
- ↑ Xu, X. and Norell, M.A. (2004). "A new troodontid dinosaur from China with avian-like sleeping posture". Nature 431 (7010): 838–841. doi:10.1038/nature02898. PMID 15483610.
- ↑ Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, Katz ME, Sugarman PJ, Cramer BS, Christie-Blick N, Pekar SF (2005). "The Phanerozoic record of global sea-level change". Science 310 (5752): 1293–8. doi:10.1126/science.1116412. PMID 16311326.
- ↑ McArthura JM, Janssenb NMM, Rebouletc S, Lengd MJ, Thirlwalle MF & van de Shootbruggef B (2007). "Palaeotemperatures, polar ice-volume, and isotope stratigraphy (Mg/Ca, δ18O, δ13C, 87Sr/86Sr): The Early Cretaceous (Berriasian, Valanginian, Hauterivian)". Palaeogeography, Palaeoclimatology, Palaeoecology 248 (3–4): 391–430. doi:10.1016/j.palaeo.2006.12.015.
- ↑ Alvarez, LW, Alvarez, W, Asaro, F, and Michel, HV (1980). "Extraterrestrial cause for the Cretaceous–Tertiary extinction". Science 208 (4448): 1095–1108. doi:10.1126/science.208.4448.1095. PMID 17783054.
- ↑ Hildebrand, Alan R.; Penfield, Glen T.; Kring, David A.; Pilkington, Mark; Zanoguera, Antonio Camargo; Jacobsen, Stein B.; Boynton, William V. (September 1991). "Chicxulub Crater; a possible Cretaceous/Tertiary boundary impact crater on the Yucatan Peninsula, Mexico". Geology 19 (9): 867–871. doi:10.1130/0091-7613(1991)019<0867:CCAPCT>2.3.CO;2. http://geology.geoscienceworld.org/cgi/content/abstract/19/9/867.
- ↑ Pope KO, Ocampo AC, Kinsland GL, Smith R (1996). "Surface expression of the Chicxulub crater". Geology 24 (6): 527–30. doi:10.1130/0091-7613(1996)024<0527:SEOTCC>2.3.CO;2. PMID 11539331.
- ↑ P, Claeys; Goderis, S (2007-09-05). "Solar System: Lethal billiards". Nature 449 (7158): 30–31. doi:10.1038/449030a. PMID 17805281.
- ↑ 124.0 124.1 edited by Christian Koeberl and Kenneth G. MacLeod. (2002). Catastrophic Events and Mass Extinctions. Geological Society of America. ISBN 0-8137-2356-6. OCLC 213836505.
- ↑ 125.0 125.1 Hofman, C, Féraud, G & Courtillot, V (2000). "40Ar/39Ar dating of mineral separates and whole rocks from the Western Ghats lava pile: further constraints on duration and age of the Deccan traps". Earth and Planetary Science Letters 180: 13–27. doi:10.1016/S0012-821X(00)00159-X.
- ↑ 126.0 126.1 126.2 Duncan, RA & Pyle, DG (1988). "Rapid eruption of the Deccan flood basalts at the Cretaceous/Tertiary boundary". Nature 333: 841–843. doi:10.1038/333841a0.
- ↑ Alvarez, W (1997). T. rex and the Crater of Doom. Princeton University Press. pp. 130–146. ISBN 978-0691016306.
- ↑ Fassett, JE, Lucas, SG, Zielinski, RA, and Budahn, JR (2001). "Compelling new evidence for Paleocene dinosaurs in the Ojo Alamo Sandstone, San Juan Basin, New Mexico and Colorado, USA" (PDF). Catastrophic events and mass extinctions, Lunar and Planetary Contribution 1053: 45–46. http://www.lpi.usra.edu/meetings/impact2000/pdf/3139.pdf. Retrieved 2007-05-18.
- ↑ Sloan, R. E., Rigby, K,. Van Valen, L. M., Gabriel, Diane (1986). "Gradual dinosaur extinction and simultaneous ungulate radiation in the Hell Creek Formation". Science 232 (4750): 629–633. doi:10.1126/science.232.4750.629. PMID 17781415. http://www.sciencemag.org/cgi/content/abstract/232/4750/629. Retrieved 2007-05-18.
- ↑ 130.0 130.1 Fastovsky, David E.; Sheehan, Peter M. (2005). "Reply to comment on "The Extinction of the dinosaurs in North America"" (pdf). GSA Today 15: 11. doi:10.1130/1052-5173(2005)015[11b:RTEOTD]2.0.CO;2. http://www.gsajournals.org/archive/1052-5173/15/7/pdf/i1052-5173-15-7-11a.pdf.
- ↑ Sullivan, RM (2003). "No Paleocene dinosaurs in the San Juan Basin, New Mexico". Geological Society of America Abstracts with Programs 35 (5): 15. http://gsa.confex.com/gsa/2003RM/finalprogram/abstract_47695.htm. Retrieved 2007-07-02.
- ↑ Dong Zhiming (1992). Dinosaurian Faunas of China. China Ocean Press, Beijing. ISBN 3-540-52084-8. OCLC 26522845.
- ↑ "Dinosaur bones 'used as medicine'". BBC News. 2007-07-06. http://news.bbc.co.uk/2/hi/asia-pacific/6276948.stm. Retrieved 2007-07-06.
- ↑ 134.0 134.1 Sarjeant, William A.S. (1997). "The earliert discoveries". In Farlow, James O.; and Brett-Surman, Michael K. (eds.). The Complete Dinosaur. Bloomington: Indiana University Press. pp. 3–11. ISBN 0-253-33349-0.
- ↑ Lhuyd, E. (1699). Lithophylacii Britannici Ichnographia, sive lapidium aliorumque fossilium Britannicorum singulari figura insignium. Gleditsch and Weidmann:London.
- ↑ Delair, J.B., and Sarjeant, W.A.S. (2002). The earliest discoveries of dinosaurs: the records re-examined. Proceedings of the Geologists' Association 113:185–197.
- ↑ Gunther, R.T. (1945). Early Science in Oxford: Life and Letters of Edward Lhuyd, volume 14. Author:Oxford.
- ↑ Buckland, W. (1824). "Notice on the Megalosaurus or great Fossil Lizard of Stonesfield." Transactions of the Geological Society of London, series 2, vol. 1: 390–396.
- ↑ Mantell, Gideon A. (1825). "Notice on the Iguanodon, a newly discovered fossil reptile, from the sandstone of Tilgate forest, in Sussex.". Philosophical Transactions of the Royal Society 115: 179–186. doi:10.1098/rstl.1825.0010. http://links.jstor.org/sici?sici=0261-0523(1825)115%3C179%3ANOTIAN%3E2.0.CO%3B2-W. Retrieved 2007-02-21.
- ↑ Sues, Hans-Dieter (1997). "European Dinosaur Hunters". In James Orville Farlow and M. K. Brett-Surman (eds.). The Complete Dinosaur. Bloomington: Indiana University Press. pp. 14. ISBN 0-253-33349-0.
- ↑ Holmes T (1996). Fossil Feud: The Bone Wars of Cope and Marsh, Pioneers in Dinosaur Science. Silver Burdett Press. ISBN 978-0382391477. OCLC 34472600.
- ↑ Torrens, H.S. (1993). "The dinosaurs and dinomania over 150 years". Modern Geology 18 (2): 257–286.
- ↑ Breithaupt, Brent H. (1997). "First golden period in the USA". In Currie, Philip J. and Padian, Kevin (eds.). The Encyclopedia of Dinosaurs. San Diego: Academic Press. pp. 347–350. ISBN 978-0122268106.
- ↑ "London. Michaelmas term lately over, and the Lord Chancellor sitting in Lincoln's Inn Hall. Implacable November weather. As much mud in the streets, as if the waters had but newly retired from the face of the earth, and it would not be wonderful to meet a Megalosaurus, forty feet long or so, waddling like an elephantine lizard up Holborne Hill." From page 1 of Dickens, Charles J.H. (1852). Bleak House. London: Bradbury & Evans.
- ↑ Glut, D.F., and Brett-Surman, M.K. (1997). Farlow, James O. and Brett-Surman, Michael K. (eds.). ed. The Complete Dinosaur. Indiana University Press. pp. 675–697. ISBN 978-0253213136.
Further reading
- Bakker, Robert T. (1986). The Dinosaur Heresies: New Theories Unlocking the Mystery of the Dinosaurs and Their Extinction. New York: Morrow. ISBN 0688042872.
- Holtz, Thomas R. Jr. (2007). Dinosaurs: The Most Complete, Up-to-Date Encyclopedia for Dinosaur Lovers of All Ages. New York: Random House. ISBN 9780375824197.
- Paul, Gregory S. (2000). The Scientific American Book of Dinosaurs. New York: St. Martin's Press. ISBN 0312262264.
- Paul, Gregory S. (2002). Dinosaurs of the Air: The Evolution and Loss of Flight in Dinosaurs and Birds. Baltimore: The Johns Hopkins University Press. ISBN 0801867630.
External links
- General
- Images
- The Science and Art of Gregory S. Paul Influential paleontologist's anatomy art and paintings
- Skeletal Drawing Professional restorations of numerous dinosaurs, and discussions of dinosaur anatomy.
- Popular
- Dinosaurs & other extinct creatures: From the Natural History Museum, a well illustrated dinosaur directory.
- Dinosaurnews (www.dinosaurnews.org) The dinosaur-related headlines from around the world. Recent news on dinosaurs, including finds and discoveries, and many links.
- Dinosauria From UC Berkeley Museum of Paleontology Detailed information – scroll down for menu.
- LiveScience.com All about dinosaurs, with current featured articles.
- Zoom Dinosaurs (www.enchantedlearning.com) From Enchanted Learning. Kids' site, info pages and stats, theories, history.
- Dinosaur genus list contains data tables on nearly every published dinosaur genus.
- LiveScience.com Giant Dinosaurs Get Downsized by LiveScience, June 21, 2009
- Technical
- Palaeontologia Electronica From Coquina Press. Online technical journal.
- Dinobase A searchable dinosaur database, from the University of Bristol, with dinosaur lists, classification, pictures, and more.
- DinoData (www.dinodata.org) Technical site, essays, classification, anatomy.
- Dinosauria On-Line (www.dinosauria.com) Technical site, essays, pronunciation, dictionary.
- Thescelosaurus! By Justin Tweet. Includes a cladogram and small essays on each relevant genera and species.
- Dinosauromorpha Cladogram From Palaeos. A detailed amateur site about all things paleo.
|
|||||||||||||||||