Dimethyltryptamine
N,N-Dimethyltryptamine (DMT) is a naturally occurring hallucinogenic drug of the tryptamine family. This drug is found not only in many plants[3], but also in trace amounts in the human body, where its natural function remains undetermined. Structurally, it is analogous to the neurotransmitter serotonin (5-HT) and other hallucinogenic tryptamines such as 5-MeO-DMT, bufotenin (5-OH-DMT), and psilocin (4-HO-DMT). DMT is created in small amounts by the human body during normal metabolism[4] by the enzyme tryptamine-N-methyltransferase. Many cultures, indigenous and modern, ingest DMT as a psychedelic, in either extracted or synthesized forms.[5] When refined, DMT is a clear to white, crystalline solid. However, DMT found on the illicit market is commonly impure and may appear yellow, orange, or salmon in color, unless special care has been taken to remove these impurities. Such impurities result from degradation or originate from plant matter from which the DMT may have been extracted.
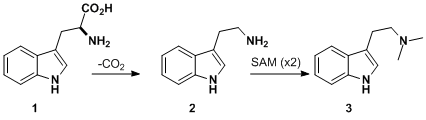
Biosynthesis
Dimethyltryptamine is an indole-alkaloid derived from the shikimate pathway. Its biosynthesis is relatively simple and summarized in the picture to the left. In plants, the tryptophan is produced endogenously where in animals the tryptophan used comes from diet. No matter the source of tryptophan, the biosynthesis begins with the decarboxylation of L-tryptophan (step 1). Once decarboxylated, tryptamine (step 2) is dimethylated by S-adenosyl-methionine (SAM) via nucleophilic attack. This reaction is mediated by tryptamine-N-methyltransferase enzyme. This produces the product (step 3). The mechanism has been proven by radio labelling of SAM with carbon-14.[6] The study found that various mammal tissues contained enzymes capable of performing the above transformation.
Chemistry

DMT Crystals
DMT is a derivative of tryptamine with two additional methyl groups at the amine nitrogen atom. DMT was first synthesized by Canadian chemist Richard Manske in 1931.[3] Natural DMT was first extracted from the plant roots of Mimosa hostilis in 1946 by Brazilian ethnobotanist and chemist Gonçalves de Lima who named it Nigerine. DMT is commonly handled and stored as a fumarate as other DMT acid salts are generally very hygroscopic and will not readily crystallize. Its freebase form, although less stable than DMT fumarate, is favored by recreational users choosing to vaporize (smoke) the chemical because it has a lower boiling point. In contrast to DMT's base, its salts are water-soluble. DMT in solution degrades relatively quickly and should be stored protected from air, light, and heat in a freezer.
Pharmacology
DMT acts as a non-selective agonist at most or all of the serotonin receptors (including 5-HT2A and 5-HT2C),[7][8][9][10] and has also been shown to possess affinity for the D1, α1-adrenergic, α2-adrenergic, imidazoline-1, sigma-1, and trace amine-associated receptors.[7][8][11] At the trace amine-associated receptor (TAAR) and the sigma-1 receptor it acts as an agonist,[11][12] whereas its efficacies at the other sites are unclear. It has also been shown to bind to the SERT and VMAT2 as a substrate (instead of inhibitor), and may act as a serotonin releasing agent.[13] The psychedelic effects of DMT can likely largely be attributed to activation of the 5-HT2A receptor,[14][15] though it cannot be ruled out whether other receptors such as 5-HT2C, sigma-1, and TAAR may also play a role.[11][12][15]
It was speculated that DMT could be an endogenous ligand for the sigma-1 receptor. In this report, the concentration of DMT needed for sigma-1 activation is about 9.4 mg/L (50 µM). This concentration is higher than the average concentration measured in brain tissue or plasma, and is also about two orders of magnitude higher than that needed to activate the 5-HT2A receptor in vitro. In humans, effective hallucinogenic doses produce peak DMT plasma concentrations ranging between 12 and 90 µg/L and with an apparent volume of distribution of 36 to 55 L.[16][17][18]
The corresponding average molar plasma concentration of DMT is therefore in the range of 0.060–0.500 µM. However, the relatively high volume of distribution of DMT indicates significant movement of the drug from plasma into tissues and several reports have described the active accumulation of DMT and other tryptamines into rat brain following peripheral administration.[19][20][21][22][23] Similar active uptake processes in human brain may plausibly concentrate DMT within neurons by several-fold or more, resulting in local concentrations in the micromolar or higher range. Interestingly, the concentrations of DMT required to occupy a significant fraction of any of its known receptor binding sites are between 1,000 and 1,000,000-fold lower than the calculated synaptic concentration of other neurotransmitters. For example, using amperometric measurements, the synaptic concentration of dopamine was estimated to reach about 75 mM.[24] DMT also activated the trace amine associated receptor TAAR1, at the tested high concentration.[25]
Psychedelic properties
DMT occurs naturally in many species of plants often in conjunction with its close chemical relatives 5-MeO-DMT and bufotenin (5-OH-DMT).[26] DMT-containing plants are commonly used in South American Shamanic practices. It is usually one of the main active constituents of the drink ayahuasca[5], however ayahuasca is sometimes brewed without plants that produce DMT. It occurs as the primary psychoactive alkaloid in several plants including Mimosa hostilis, Diplopterys cabrerana, and Psychotria viridis. DMT is found as a minor alkaloid in snuff made from Virola bark resin in which 5-MeO-DMT is the main active alkaloid.[26] DMT is also found as a minor alkaloid in the beans of Anadenanthera peregrina and Anadenanthera colubrina used to make Yopo and Vilca snuff in which bufotenin is the main active alkaloid.[26][27] Psilocybin and psilocin, active chemicals in many psychedelic mushrooms, are structurally very similar to DMT.[28]
The psychotropic effects of DMT were first studied scientifically by the Hungarian chemist and psychologist Dr. Stephen Szára who performed research with volunteers in the mid-1950s. Szára, who later worked for the US National Institutes of Health, had turned his attention to DMT after his order for LSD from the Swiss company Sandoz Laboratories was rejected on the grounds that the powerful psychotropic could be dangerous in the hands of a communist country.[29]

DMT during various stages of purification in an illegal drug laboratory in Los Angeles
DMT can produce powerful entheogenic experiences including intense visuals, euphoria, even true hallucinations (perceived extensions of reality). DMT is generally not active orally unless it is combined with a monoamine oxidase inhibitor (MAOI) such as a reversible inhibitor of monoamine oxidase A (RIMA), e.g., harmaline. Uninhibited, the human body metabolizes DMT too rapidly for oral administration to be effective. Other means of ingestion such as smoking or injecting the drug can produce powerful hallucinations and entheogenic activity for a short time (usually less than half an hour), as the DMT reaches the brain before it can be metabolized by the body's natural monoamine oxidase. Taking a MAOI prior to smoking or injecting DMT prolongs and potentiates the effects.[30]
Inhalation
A standard dose for smoked DMT is between 15–60 mg. This is generally smoked in a few successive breaths. The effects last for a short period of time, usually 5 to 15 minutes, dependent on the dose. The onset after inhalation is very fast (less than 45 seconds) and peak effects are reached within a minute. In the 1960s, some reportedly referred to DMT as "the businessman's trip"[31] because of the relatively short duration of vaporized, insufflated, or injected DMT. DMT is commonly vaporized in glass pipes such as those used with crack cocaine and methamphetamine. Improvised smoking devices can be constructed from light bulbs. The vapor is sometimes described as harsh, and some users even compare its flavor to that of burning plastic. Some users elect to combine it with cannabis, parsley, mullein, or other plants in an attempt to improve flavor and reduce harshness. Combining DMT with plant matter or depositing it upon a substrate of ash also facilitates use of an ordinary pipe, a bong, or a vaporizer.
Insufflation
Insufflating DMT (commonly as a freebase or fumarate) requires a higher dose than does inhalation. The duration is markedly increased, and some users report diminished euphoria but an intensified otherworldly experience .
Injection
Injected DMT produces an experience that is similar to inhalation in duration, intensity, and characteristics.
In a study conducted from 1990 through 1995, University of New Mexico psychiatrist Rick Strassman found that some volunteers injected with high doses of DMT had experiences with a perceived alien entity. Usually, the reported entities were experienced as the inhabitants of a perceived independent reality the subjects reported visiting while under the influence of DMT.[29] In a September, 2009, interview with Examiner.com, Strassman described the effects on participants in the study: "Subjectively, the most interesting results were that high doses of DMT seemed to allow the consciousness of our volunteers to enter into non-corporeal, free-standing, independent realms of existence inhabited by beings of light who oftentimes were expecting the “volunteers,” and with whom the volunteers interacted. While “typical” near-death and mystical states occurred, they were relatively rare."
Oral ingestion
DMT is broken down by the digestive enzyme monoamine oxidase and is practically inactive if taken orally, unless combined with a MAOI. The traditional South American ayahuasca, or yage, is a tea mixture containing DMT and a MAOI.[5] There are a variety of recipes to this brew, but most commonly it is simply the leaves of Psychotria viridis (the source of DMT) and the vine Banisteriopsis caapi (the source of MAOI). It has often puzzled researchers how the natives managed to find this combination without modern analytical chemistry. Other DMT containing plants, including Diplopterys cabrerana, are sometimes used in ayahuasca in different areas of South America. Two common sources in the western US are Reed canary grass (Phalaris arundinacea) and Harding grass (Phalaris aquatica). These invasive grasses contain low levels of DMT and other alkaloids. In addition, Jurema (Mimosa hostilis) shows evidence of DMT content: the pink layer in the bark of this vine contains a high concentration of N,N-DMT.
Taken orally with an appropriate MAOI, DMT produces a long lasting (over 3 hour), slow, deep metaphysical experience similar to that of psilocybin mushrooms, but more intense.[5] MAOIs should be used with extreme caution as they can have lethal complications with some prescription drugs such as SSRI antidepressants, some over-the-counter drugs,[32] and many common foods.[33]
Induced DMT experiences can include profound time-dilation, visual and auditory illusions, and other experiences that, by most firsthand accounts, defy verbal or visual description. Some users report intense erotic imagery and sensations and utilize the drug in a ritual sexual context.[5][34][35]
Distinguish from 5-MeO-DMT
5-MeO-DMT, a psychedelic drug structurally similar to N,N-DMT, is sometimes referred to as DMT through ignorance or by abbreviation. As a white, crystalline solid, it is also similar in appearance to DMT. However, it is considerably more potent (5-MeO-DMT typical smoked dose: 5–20 mg), and care should be taken to clearly differentiate between the two drugs to avoid accidental overdose.[36]
Side effects
When DMT is vaporized, the inhaled vapor feels harsh to a minority of users. According to a "Dose-response study of N,N-dimethyltryptamine in humans" by Rick Strassman, "Dimethyltryptamine dose slightly elevated blood pressure, heart rate, pupil diameter, and rectal temperature, in addition to elevating blood concentrations of beta-endorphin, corticotropin, cortisol, and prolactin. Growth hormone blood levels rose equally in response to all doses of DMT, and melatonin levels were unaffected."[37]
Speculation

DMT crystal at 400x magnification.
Several speculative and yet untested hypotheses suggest that endogenous DMT, produced in the human brain, is involved in certain psychological and neurological states. DMT is naturally produced in small amounts in the brain and other tissues of humans and other mammals.[38] It may play a role in mediating the visual effects of natural dreaming, and also near-death experiences, religious visions and other mystical states.[39] A biochemical mechanism for this was proposed by the medical researcher J. C. Callaway, who suggested in 1988 that DMT might be connected with visual dream phenomena, where brain DMT levels are periodically elevated to induce visual dreaming and possibly other natural states of mind.[40] A new hypothesis proposed is that in addition to being involved in altered states of consciousness, endogenous DMT may be involved in the creation of normal waking states of consciousness. It is proposed that DMT and other endogenous hallucinogens mediate their neurological abilities by acting as neurotransmitters at a sub class of the trace amine receptors; a group of receptors found in the CNS where DMT and other hallucinogens have been shown to have activity. Wallach further proposes that in this way waking consciousness can be thought of as a controlled psychedelic experience. It is when the control of these systems becomes loosened and their behavior no longer correlates with the external world that the altered states arise.[41]
Dr. Rick Strassman, while conducting DMT research in the 1990s at the University of New Mexico, advanced the theory that a massive release of DMT from the pineal gland prior to death or near death was the cause of the near death experience (NDE) phenomenon. Several of his test subjects reported NDE-like audio or visual hallucinations. His explanation for this was the possible lack of panic involved in the clinical setting and possible dosage differences between those administered and those encountered in actual NDE cases. Several subjects also reported contact with 'other beings', alien like, insectoid or reptilian in nature, in highly advanced technological environments[29] where the subjects were 'carried', 'probed', 'tested', 'manipulated', 'dismembered', 'taught', 'loved' and even 'raped' by these 'beings' (one could note the strong similarities of these bodily tests/invasions in other psychedelic experiences throughout time, outlined in Graham Hancock's "Supernatural"[42]). Strassman has speculated that DMT is made in the pineal gland, largely because the necessary constituents (see methyltransferases) needed to make DMT are found in the pineal gland in substantially greater concentrations than any other part of the body. However, there is no scientific proof of this.
In the 1950s, the endogenous production of psychoactive agents was considered to be a potential explanation for the hallucinatory symptoms of some psychiatric diseases as the transmethylation hypothesis[43] (see also adrenochrome), though this hypothesis does not account for the natural presence of endogenous DMT in otherwise normal humans, rats and other laboratory animals.
Writers on DMT include Terence McKenna, Jeremy Narby and Graham Hancock. In his writings and speeches, Terence McKenna recounts encounters with entities he sometimes describes as "Self-Transforming Machine Elves" among other phrases. McKenna believed DMT to be a tool that could be used to enhance communication and allow for communication with other-worldly entities. Other users report visitation from external intelligences attempting to impart information. These Machine Elf experiences are said to be shared by only a minority of DMT users and some people report never seeing or experiencing anything of that nature.
Legal status
International Law
DMT is classified as a Schedule I drug under the UN 1971 Convention on Psychotropic Substances, meaning that use of DMT is supposed to be restricted to scientific research and medical use and international trade in DMT is supposed to be closely monitored. Natural materials containing DMT, including ayahuasca, are explicitly not regulated under the 1971 Psychotropic Convention.[44]
United States
DMT is classified in the United States as a Schedule I drug under the Controlled Substances Act of 1970.
In December 2004, the Supreme Court lifted a stay thereby allowing the Brazil-based União do Vegetal (UDV) church to use a decoction containing DMT in their Christmas services that year. This decoction is a "tea" made from boiled leaves and vines, known as hoasca within the UDV, and ayahuasca in different cultures. In Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, the Supreme Court heard arguments on November 1, 2005 and unanimously ruled in February 2006 that the U.S. federal government must allow the UDV to import and consume the tea for religious ceremonies under the 1993 Religious Freedom Restoration Act.
In September, 2008, the three Santo Daime churches filed suit in federal court to gain legal status to import DMT-containing ayahuasca tea. The case, Church of the Holy Light of the Queen v. Mukasey[45], presided over by Judge Owen M. Panner, was ruled in favor of the Santo Daime church. As of March 21, 2009 a federal judge says members of the church in Ashland can import, distribute and brew ayahuasca. U.S. District Judge Owen Panner issued a permanent injunction barring the government from prohibiting or penalizing the sacramental use of "Daime tea." Panner's order said activities of The Church of the Holy Light of the Queen are legal and protected under freedom of religion. His order prohibits the federal government from interfering and prosecuting church members who follow a list of regulations set out in his order.[46][47]
Canada
DMT is classified in Canada as a Schedule III drug.
France
DMT, along with most of its plant sources, is classified in France as a stupéfiant (Narcotic).
United Kingdom
DMT is classified in the United Kingdom as a Class A drug.
New Zealand
DMT is classified as a Class A drug in New Zealand.
Culture
In South America there are a number of indigenous traditions and more recent religious movements based on the use of ayahuasca, usually in an animistic context that may be mixed with Christian imagery. There are four main branches using DMT-MAOI based sacraments in South America:
- Amazonian Peoples. There are many indigenous cultures in South America, mostly in the Upper Amazonn Basin whose traditional religious practices include the use of ayahuasca. These are the oldest cultures in the whole of South America that continue to use ayahuasca or analogue brews, such as the ones made from Jurema in the Pernambuco, near Recife or Iquitos in Peru.
- Santo Daime ("Saint Give Unto Me") and Barquinha ("Little Boat"). A syncretic religion from Brazil. The former was founded by Raimundo Irineu Serra in the early 1930s, as an esoteric Christian religion with shamanic tendencies. The Barquinha was derived from this one.
- União do Vegetal (Union of Vegetal or UDV). Another Christian ayahuasca religion from Brazil, is a single unified organization with a democratic structure.
- Neo-shamans. There are some self-styled shamanic facilitators in Brazil and other South American countries that use ayahuasca or analogous brews in their rituals and séances.
See also
References
- ↑ Häfelinger, Günter; Nimtz, Manfred; Horstmann, Volker; Benz, Thomas (1999), "Untersuchungen zur Trifluoracetylierung der Methylderivate von Tryptamin und Serotonin mit verschiedenen Derivatisierungsreagentien: Synthesen, Spektroskopie sowie analytische Trennungen mittels Kapillar-GC Trifluoracetylation of Methylated -Derivatives of Tryptamine and Serotonine by Different Reagents: Synthesis, Spectroscopic Characterizations, and Separations by Capillary-Gas-Chromatography", Zeitschrift für Naturforschung - Section B Journal of Chemical Sciences 54 (3): 397–414, http://www.znaturforsch.com/ab/v54b/c54b.htm#No3
- ↑ E. Corothie, T. Nakano (1969). "CONSTITUENTS OF THE BARK OF VIROLA SEBIFERA". Planta Medica 17 (2): 184-188. doi:10.1055/s-0028-1099844. PMID 5792479. https://www.thieme-connect.de/DOI/DOI?10.1055/s-0028-1099844. Retrieved 3 September 2010.
- ↑ 3.0 3.1 Jeremy Bigwood and Jonathan Ott (1977): "DMT", Head Magazine
- ↑ Barker SA, Monti JA, Christian ST (1981). "N, N-dimethyltryptamine: an endogenous hallucinogen". Int. Rev. Neurobiol. 22: 83–110. doi:10.1016/S0074-7742(08)60291-3. PMID 6792104.
- ↑ 5.0 5.1 5.2 5.3 5.4 Salak, Kira. ""HELL AND BACK"". National Geographic Adventure. http://www.kirasalak.com/Peru.html.
- ↑ Mandel LR, Prasad R, Lopez-Ramos B, Walker RW (January 1977). "The biosynthesis of dimethyltryptamine in vivo". Res. Commun. Chem. Pathol. Pharmacol. 16 (1): 47–58. PMID 14361.
- ↑ 7.0 7.1 Ray TS; Manzoni, Olivier Jacques (2010). "Psychedelics and the human receptorome". PLoS ONE 5 (2): e9019. doi:10.1371/journal.pone.0009019. PMID 20126400. PMC 2814854. http://dx.plos.org/10.1371/journal.pone.0009019.
- ↑ 8.0 8.1 Pierce PA, Peroutka SJ (1989). "Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex". Psychopharmacology 97 (1): 118–22. doi:10.1007/BF00443425. PMID 2540505.
- ↑ McKenna DJ, Peroutka SJ (October 1989). "Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(-)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin". The Journal of Neuroscience : the Official Journal of the Society for Neuroscience 9 (10): 3482–90. PMID 2795135. http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=2795135.
- ↑ Smith RL, Canton H, Barrett RJ, Sanders-Bush E (November 1998). "Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors". Pharmacology, Biochemistry, and Behavior 61 (3): 323–30. doi:10.1016/S0091-3057(98)00110-5. PMID 9768567. http://linkinghub.elsevier.com/retrieve/pii/.
- ↑ 11.0 11.1 11.2 Wallach JV (January 2009). "Endogenous hallucinogens as ligands of the trace amine receptors: a possible role in sensory perception". Medical Hypotheses 72 (1): 91–4. doi:10.1016/j.mehy.2008.07.052. PMID 18805646. http://linkinghub.elsevier.com/retrieve/pii/S0306-9877(08)00398-8.
- ↑ 12.0 12.1 Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE (February 2009). "The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator". Science (New York, N.Y.) 323 (5916): 934–7. doi:10.1126/science.1166127. PMID 19213917. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=19213917.
- ↑ Cozzi NV, Gopalakrishnan A, Anderson LL, et al. (December 2009). "Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter". Journal of Neural Transmission (Vienna, Austria : 1996) 116 (12): 1591–9. doi:10.1007/s00702-009-0308-8. PMID 19756361.
- ↑ Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (December 1998). "Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action". Neuroreport 9 (17): 3897–902. doi:10.1097/00001756-199812010-00024. PMID 9875725. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0959-4965&volume=9&issue=17&spage=3897.
- ↑ 15.0 15.1 Nichols DE (February 2004). "Hallucinogens". Pharmacology & Therapeutics 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703. http://linkinghub.elsevier.com/retrieve/pii/S0163725803001657.
- ↑ Callaway JC, McKenna DJ, Grob CS, et al. (June 1999). "Pharmacokinetics of Hoasca alkaloids in healthy humans". J Ethnopharmacol 65 (3): 243–56. doi:10.1016/S0378-8741(98)00168-8. PMID 10404423.
- ↑ Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ (July 2003). "Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics". J. Pharmacol. Exp. Ther. 306 (1): 73–83. doi:10.1124/jpet.103.049882. PMID 12660312.
- ↑ Yritia M, Riba J, Ortuño J, et al. (November 2002). "Determination of N,N-dimethyltryptamine and beta-carboline alkaloids in human plasma following oral administration of Ayahuasca". J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 779 (2): 271–81. doi:10.1016/S1570-0232(02)00397-5. PMID 12361741.
- ↑ Barker SA, Beaton JM, Christian ST, Monti JA, Morris PE (August 1982). "Comparison of the brain levels of N,N-dimethyltryptamine and alpha, alpha, beta, beta-tetradeutero-N-N-dimethyltryptamine following intraperitoneal injection. The in vivo kinetic isotope effect". Biochem. Pharmacol. 31 (15): 2513–6. doi:10.1016/0006-2952(82)90062-4. PMID 6812592.
- ↑ Sangiah S, Gomez MV, Domino EF (December 1979). "Accumulation of N,N-dimethyltryptamine in rat brain cortical slices". Biol. Psychiatry 14 (6): 925–36. PMID 41604.
- ↑ Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR (May 1987). "In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat". Biochem. Pharmacol. 36 (9): 1509–12. doi:10.1016/0006-2952(87)90118-3. PMID 3472526.
- ↑ Takahashi T, Takahashi K, Ido T, et al. (December 1985). "[11C]-labeling of indolealkylamine alkaloids and the comparative study of their tissue distributions". Int J Appl Radiat Isot 36 (12): 965–9. doi:10.1016/0020-708X(85)90257-1. PMID 3866749.
- ↑ Yanai K, Ido T, Ishiwata K, et al. (1986). "In vivo kinetics and displacement study of a carbon-11-labeled hallucinogen, N,N-[11C]dimethyltryptamine". Eur J Nucl Med 12 (3): 141–6. PMID 3489620.
- ↑ Colliver TL, Pyott SJ, Achalabun M, Ewing AG (July 2000). "VMAT-Mediated changes in quantal size and vesicular volume". J. Neurosci. 20 (14): 5276–82. PMID 10884311.
- ↑ Bunzow JR, Sonders MS, Arttamangkul S (2001). "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor.". Mol. Pharmacol. 60 (6): 1181–8. PMID 11723224.
- ↑ 26.0 26.1 26.2 Anadenanthera: Visionary Plant Of Ancient South America By Constantino Manuel Torres, David B. Repke, 2006, ISBN 0789026422
- ↑ Pharmanopo-Psychonautics: Human Intranasal, Sublingual, Intrarectal, Pulmonary and Oral Pharmacology of Bufotenine by Jonathan Ott, The Journal of Psychoactive Drugs, September 2001
- ↑ Relation between DMT and other related compounds http://deoxy.org/t_thc.htm
- ↑ 29.0 29.1 29.2 R.J. Strassman. "Chapter summaries". DMT: The Spirit Molecule. http://rickstrassman.com/dmt/chaptersummaries.html. Retrieved 2007-01-13.
- ↑ http://www.erowid.org/chemicals/dmt/dmt.shtml
- ↑ Haroz, R; Greenberg, M (2005). "Emerging Drugs of Abuse". Medical Clinics of North America 89 (6): 1259. doi:10.1016/j.mcna.2005.06.008. PMID 16227062.
- ↑ Callaway J.C. and Grob C.S. (1998). Ayahuasca preparations and serotonin reuptake inhibitors: a potential combination for adverse interaction. Journal of Psychoactive Drugs 30(4): 367-369.
- ↑ "MAOIs and Diet". http://www.mayoclinic.com. http://www.mayoclinic.com/health/maois/HQ01575. Retrieved 2009-02-22.
- ↑ "2C-B, DMT, You and Me". Maps. http://www.maps.org/news-letters/v12n1/12125set.html. Retrieved 2007-01-13.
- ↑ "Entheogens & Visionary Medicine Pages". Miqel.com. http://www.miqel.com/entheogens/psychedelics_entheogens.html. Retrieved 2007-08-17.
- ↑ http://www.erowid.org/chemicals/5meo_dmt/5meo_dmt_dose.shtml
- ↑ R.J. Strassman and C.R. Qualls (February 1994). "Dose-response study of N,N-dimethyltryptamine in humans". Arch Gen Psychiatry 51 (2): 85–97. PMID 8297216.
- ↑ "Erowid DMT Vault: Journal Articles & Abstracts". http://www.erowid.org/chemicals/dmt/dmt_journal.shtml. Retrieved 2007-09-05.
- ↑ http://www.npr.org/templates/story/story.php?storyId=104240746&sc=fb&cc=fp
- ↑ Callaway J (1988). "A proposed mechanism for the visions of dream sleep". Med Hypotheses 26 (2): 119–24. doi:10.1016/0306-9877(88)90064-3. PMID 3412201.
- ↑ Wallach J (2008). "Endogenous hallucinogens as ligands of the trace amine receptors: A possible role in sensory perception". Med Hypotheses in print (in print): in print. doi:10.1016/j.mehy.2008.07.052. PMID 18805646.
- ↑ http://www.amazon.com/Supernatural-Meetings-Ancient-Teachers-Mankind/dp/1932857400
- ↑ Osmund H and Smythies JR (1952). Schizophrenia: A new approach. Journal of Mental Science 98:309-315.
- ↑ Erowid
- ↑ Church of the Holy Light of the Queen v. Mukasey
- ↑ Pharma-Law E-News
- ↑ Portland Tribune
External links
|
Recreational drug use |
|
| Major Recreational Drugs |
|
|
|
|
|
Depressants
|
|
|
|
|
|
|
|
Entactogens
|
|
|
|
Hallucinogens
|
|
Psychedelics
|
|
|
|
Dissociatives
|
|
|
|
Deliriants
|
|
|
|
|
|
|
|
 |
|
| Culture and Related Topics |
|
|
420 · Stoner Film · Spiritual Use of Cannabis · Medical Cannabis · Cannabis Cultivation · Cannabis smoking
|
|
|
Psychedelic
|
Art · Drug · Experience · Literature · Music
|
|
|
Other
|
Counterculture of the 1960s · Club Drug · Dance Party · Drug Tourism · Drug Paraphernalia · Hippie · Party and Play · Poly Drug Use · Rave · Self-medication · Sex and Drugs · Spiritual use of drugs |
|
|
| Problems with Drug Use |
Abuse · Addiction (Prevention · Opioid Replacement Therapy · Rehabilitation · Responsible Use) · Drug-related crime · Illegal Trade · Overdose |
|
| Legality of Drug Use |
|
International
|
1961 Narcotic Drugs · 1971 Psychotropic Substances · 1988 Drug Trafficking
|
|
|
State Level
|
Drug Policy (Prohibition · Supply reduction · Decriminalization) · Policy Reform (Liberalization · Harm Reduction · Demand Reduction)
|
|
|
Other
|
Designer Drug · Drug Possession · Drug Test · Hard and Soft Drugs · Narc · War on Drugs · Mexican Drug War |
|
|
| Lists of Countries by... |
Alcohol Consumption · Cannabis Legality (Annual Use · Lifetime Use) · Cocaine Use · Opiate Use · Cigarette Consumption
|
|
|
Neurotransmitters |
|
| Amino acids |
|
|
| Endocannabinoids |
2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide
|
|
| Gasotransmitters |
|
|
| Monoamines |
|
|
| Purines |
|
|
| Trace amines |
3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
|
|
| Others |
|
|
See also Template:Neuropeptides
|
|
anat(h,r,t,,b,l,s)/phys/devp///nttm/
|
noco/auto/cong/tumr, sysi/, injr
|
|
|
|
|
|
Hallucinogens |
|
Psychedelics
5-HT2AR agonists |
Lysergamides: AL-LAD • ALD-52 • BU-LAD • CYP-LAD • DAM-57 • Diallyllysergamide • Ergometrine (Ergonovine, Ergobasine) • ETH-LAD • LAE-32 • LSA (Ergine, Lysergamide) • LSD • LSH • LPD-824 • LSM-775 • Lysergic Acid 2-Butyl Amide • Lysergic Acid 2,4-Dimethylazetidide • Methylergometrine • Methylisopropyllysergamide • Methysergide • MLD-41 • PARGY-LAD • PRO-LAD;
Phenethylamines: Aleph • 2C-B • 2C-B-FLY • 2CBFly-NBOMe • 2C-C • 2C-D • 2CD-5EtO • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-O • 2C-O-4 • 2C-P • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • 2CBCB-NBOMe • 25B-NBOMe • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 3C-E • 3C-P • Br-DFLY • DESOXY • DMMDA • DMMDA-2 • DOB • DOC • DOEF • DOET • DOF • DOI • DOM • DON • DOPR • DOTFM • Escaline • Ganesha • HOT-2 • HOT-7 • HOT-17 • IAP • Isoproscaline • Jimscaline • Lophophine • MDA • MDEA • MDMA • MMA • MMDA • MMDA-2 • MMDA-3a • MMDMA • Macromerine • Mescaline • Methallylescaline • Proscaline • TCB-2 • TFMFly • TMA;
Piperazines: pFPP • TMFPP;
Tryptamines: 1-Methyl-5-methoxy-diisopropyltryptamine • 2,N,N-TMT • 4,N,N-TMT • 4-HO-5-MeO-DMT • 4-Acetoxy-DET • 4-Acetoxy-DIPT • 4-Acetoxy-DMT • 4-Acetoxy-DPT • 4-Acetoxy-MiPT • 4-HO-DPT • 4-HO-MET • 4-Propionyloxy-DMT • 4-Hydroxy-N-Methyl-(α,N-trimethylene)tryptamine • 5-Me-MIPT • 5-N,N-TMT • 5-AcO-DMT • 5-MeO-2,N,N-TMT • 5-MeO-4,N,N-TMT • 5-MeO-α,N,N-TMT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DALT • 5-MeO-DET • 5-MeO-DIPT • 5-MeO-DMT • 5-MeO-DPT • 5-MeO-EiPT • 5-MeO-MET • 5-MeO-MIPT • 5-Methoxy-N-methyl-(α,N-trimethylene)tryptamine • 7,N,N-TMT • α,N,N-TMT • α-ET • α-MT • AL-37350A • Baeocystin • Bufotenin • DALT • DBT • DET • DIPT • DMT • DPT • EiPT • Ethocin • Ethocybin • Iprocin • MET • Miprocin • MIPT • Norbaeocystin • PiPT • Psilocin • Psilocybin;
Others: AL-38022A • Ibogaine • Noribogaine • Voacangine
|
|
Dissociatives
NMDAR antagonists |
Adamantanes: Amantadine • Memantine • Rimantadine;
Arylcyclohexylamines: 3-MeO-PCP • 4-MeO-PCP • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Methoxetamine • Neramexane • Phencyclidine • PCPr • Rolicyclidine • Tenocyclidine • Tiletamine;
Morphinans: Dextrallorphan • Dextromethorphan • Dextrorphan • Methorphan (Racemethorphan) • Morphan (Racemorphan);
Others: 2-MDP • 8A-PDHQ • Aptiganel • Dexoxadrol • Dizocilpine (MK-801) • Etoxadrol • Ibogaine • Midafotel • NEFA • Nitrous Oxide • Noribogaine • Perzinfotel • Remacemide • Selfotel • Xenon
|
|
Deliriants
mAChR antagonists |
3-Quinuclidinyl benzilate • Atropine • Benactyzine • Benzatropine • Benzydamine • Biperiden • Brompheniramine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • Chlorpheniramine • Chloropyramine • Clemastine • CS-27349 • Cyclizine • Cyproheptadine • Dicyclomine (Dicycloverine) • Dimenhydrinate • Diphenhydramine • Ditran • Doxylamine • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Flavoxate • Hydroxyzine • Hyoscyamine • Meclizine • Myristicin • N-Ethyl-3-piperidyl benzilate • N-Methyl-3-piperidyl benzilate • Pyrilamine • Orphenadrine • Oxybutynin • Pheniramine • Phenyltoloxamine • Procyclidine • Promethazine • Scopolamine (Hyoscine) • Tolterodine • Trihexyphenidyl • Tripelennamine • Triprolidine • WIN-2299
|
|
| Miscellaneous |
|
|
Cannabinol • CP-47,497 • CP-55,244 • CP-55,940 • DMHP • HU-210 • JWH-018 • JWH-030 • JWH-073 • JWH-081 • JWH-200 • JWH-250 • Nabilone • Nabitan • Nantradol • Parahexyl • THC (Dronabinol) • WIN-55,212-2
|
|
|
D2R agonists
|
|
|
|
|
|
|
|
|
|
|
|
|
2-EMSB • 2-MMSB • Alazocine • Bremazocine • Butorphanol • Cyclazocine • Cyprenorphine • Dextrallorphan • Dezocine • Enadoline • Herkinorin • HZ-2 • Ibogaine • Ketazocine • Metazocine • Nalbuphine • Nalorphine • Noribogaine • Pentazocine • Phenazocine • Salvinorin A • Spiradoline • Tifluadom • U-50,488 • U-69,593
|
|
|
|
|
|
|
Others
|
Efavirenz • Glaucine • Isoaminile
|
|
|
|
Adrenergics |
|
|
Receptor ligands |
|
|
α1
|
Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.
|
|
|
α2
|
Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.
|
|
|
|
Agonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • N-Isopropyloctopamine • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • Xipranolol
|
|
|
|
|
Reuptake inhibitors |
|
|
NET
|
Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tramadol • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine ( Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH
|
3,4-Dihydroxystyrene
|
|
|
TH
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
DBH
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
PNMT
|
CGS-19281A • SKF-64139 • SKF-7698
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.
|
|
|
COMT
|
Entacapone • Tolcapone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Dopaminergics |
|
|
Receptor ligands |
|
|
Agonists
|
Adamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635
|
|
|
Antagonists
|
Typical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Ocaperidone • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,566 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • Yohimbine
|
|
|
|
|
Reuptake inhibitors |
|
|
|
|
DAT inhibitors
|
Piperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane ( 123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tripelennamine
|
|
|
|
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH inhibitors
|
3,4-Dihydroxystyrene
|
|
|
TH inhibitors
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD / DDC inhibitors
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
|
|
|
COMT inhibitors
|
Entacapone • Tolcapone
|
|
|
DBH inhibitors
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity Enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Serotonergics |
|
|
5-HT1 receptor ligands |
|
|
5-HT1A
|
Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • Lu AA21004 • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92016A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • Xylamidine
|
|
|
5-HT1B
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • Yohimbine
|
|
|
5-HT1D
|
Agonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • Ziprasidone
|
|
|
5-HT1E
|
Agonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/Methiothepin
|
|
|
5-HT1F
|
Agonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin
|
|
|
|
|
5-HT2 receptor ligands |
|
|
|
5-HT2A
|
Agonists: Lysergamides: ALD-52 • Ergonovine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Methysergide; Phenethylamines: 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • Yohimbine
|
|
|
5-HT2B
|
Agonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • Yohimbine
|
|
|
5-HT2C
|
Agonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Lu AA24530 • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine
|
|
|
|
|
5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands |
|
|
|
5-HT3
|
Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • Lu AA21004 • Lu AA24530 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Thujone • Xenon
|
|
|
5-HT4
|
Agonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Zacopride; Others: 5-MT • BIMU-8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • TD-5108
Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186
|
|
|
5-HT5A
|
Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.
|
|
|
5-HT6
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • N-Methyl-5-HT • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • EGIS-12233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro 04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457
|
|
|
5-HT7
|
Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507
|
|
|
|
|
Reuptake inhibitors |
|
|
SERT
|
Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lu AA21004 • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Vilazodone • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorpheniramine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Meperidine (Pethidine) • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefrine • Roxindole • SB-649,915 • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Aminoindanes: 5-IAI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Diethylcathinone • Dimethylcathinone • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • NAP • Norfenfluramine • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pFPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • Viqualine
|
|
|
|
Enzyme inhibitors |
|
|
|
|
TPH
|
AGN-2979 • Fenclonine
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Reuptake enhancers: Tianeptine
|
|
|
|
|
Tryptamines |
|
2-Methyl-5-HT • 4-Acetoxy-DET • 4-Acetoxy-DIPT • 4-Acetoxy-DMT • 4-HO-αMT • 4-HO-DIPT • 4-HO-MET • 4-MeO-DMT • 4-Methyl-αET • 4-Methyl-αMT • 5-Benzyloxytryptamine • 5-Bromo-DMT • 5-Carboxamidotryptamine • 5-Fluoro-αMT • 5-HO-αMT • 5-HTP • 5-Fluoro-DMT • 5-Methyl-DMT • 5-Methoxytryptamine • 5-MeO-7,N,N-TMT • 5-MeO-αET • 5-MeO-αMT • 5-MeO-DALT • 5-MeO-DET • 5-MeO-DIPT • 5-MeO-DMT • 5-MeO-DPT • 5-MeO-MIPT • 5,7-Dihydroxytryptamine • 7-Methyl-αET • 7-Methyl-DMT • αET • αMT • Aeruginascin • AL-37350A • BW-723C86 • Baeocystin • Bufotenidine • Bufotenin • DALT • Desformylflustrabromine • DET • DiPT • DMT • DPT • Ethocybin • EiPT • EMDT • Ethocin • FGIN-127 • FGIN-143 • Ibogaine • Iprocin • MET • MiPT • Miprocin • Melatonin • MS-245 • NAS • NMT • Norbaeocystin • Normelatonin • Oxypertine • PiPT • Psilocin • Psilocybin • Rizatriptan • Serotonin • Sumatriptan • Tryptamine • Tryptophan • Yohimbine • Yuremamine • Zolmitriptan
|
|
|
Drugs from TiHKAL |
|
AL-LAD • DBT • DET • DiPT • 5-MeO-α-MT • DMT • 2,α-DMT • α,N-DMT • DPT • EiPT • α-ET • ETH-LAD • Harmaline • Harmine • 4-HO-DBT • 4-HO-DET • 4-HO-DiPT • 4-HO-DMT • 5-HO-DMT • 4-HO-DPT • 4-HO-MET • 4-HO-MiPT • 4-HO-MPT • 4-HO-pyr-T • Ibogaine • LSD • MBT • 4,5-MDO-DiPT • 5,6-MDO-DiPT • 4,5-MDO-DMT • 5,6-MDO-DMT • 5,6-MDO-MiPT • 2-Me-DET • 2-Me-DMT • Melatonin • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 4-MeO-MiPT • 5-MeO-MiPT • 5,6-MeO-MiPT • 5-MeO-NMT • 5-MeO-pyr-T • 6-MeO-THH • 5-MeO-TMT • 5-MeS-DMT • MiPT • α-MT • NET • NMT • PRO-LAD • pyr-T • Tryptamine • Tetrahydroharmine • α,N,O-TMS
|
|