Diels–Alder reaction
| Diels–Alder reaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Named after | Otto Paul Hermann Diels Kurt Alder |
||||||||
| Reaction type | Cycloaddition | ||||||||
| Reaction | |||||||||
.png)
|
|||||||||
| Identifiers | |||||||||
| RSC ontology ID | RXNO:0000006 |
||||||||
The Diels-Alder reaction is an organic chemical reaction (specifically, a cycloaddition) between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system.[1][2][3] The reaction can proceed even if some of the atoms in the newly-formed ring are not carbon. Some of the Diels-Alder reactions are reversible; the decomposition reaction of the cyclic system is then called the Retro-Diels-Alder. For example, Retro-Diels-Alder compounds are commonly observed when a Diels Alder product is analyzed via mass spectrometry.
Otto Paul Hermann Diels and Kurt Alder first documented the novel reaction in 1928 for which they were awarded the Nobel Prize in Chemistry in 1950 for their work on the eponymous reaction.
The Diels-Alder reaction is generally considered the "Mona Lisa" of reactions in organic chemistry since it requires very little energy to create a cyclohexene ring, which is useful in many other organic reactions.[4][5][6][7]
Contents |
Reaction mechanism
The reaction occurs via a single transition state, which has a smaller volume than either the starting materials or the product. It is an associative type of reaction, and it is sped up by very high pressures. Diels-Alder is an example of a pericyclic reaction.
Some free-radical versions of this reaction have been observed, though these are not Diels-Alder reactions as the stereochemistry at the carbons is scrambled. These are step-wise reactions of the free radicals which form the new bonds in at least two steps. An example of this type of reaction is the reaction of selenobenzophenone with a 1,3-diene (See: thioketones).
The diene
The diene component in the Diels-Alder reaction can be open-chain or cyclic and it can have many different kinds of substituents.[8] There is only one limitation: it must be able to exist in the s-cis conformation. Butadiene[9] itself normally prefers the s-trans conformation, with the two double bonds as far away from each other as possible. If there are substituents larger than hydrogen then steric hindrance may influence the relative stabilities of the conformations. For simple cases, the barrier to rotation about the central bond is small and rotation to the less favourable but reactive s-cis conformation is rapid. Cyclic dienes that are permanently in the s-cis conformation are exceptionally reactive in Diels-Alder reactions (cyclopentadiene is a classic example), while cyclic dienes that are permanently in the s-trans conformation and cannot adopt the s-cis conformation will not undergo the Diels-Alder reaction at all. An especially reactive diene is Danishefsky’s diene.
Dendralenes are a new class of experimental DA dienes.
Unstable dienes, such as o-quinodimethane, can be generated in situ. Aromatic stabilization in the product of a DA reaction using such a diene is, in some cases, the reason behind the very high reactivity and lack of stability of such diene. The use of such unstable dienes is advantageous, despite the trouble, in that the products will contain newly formed aromatic six-membered rings.
Benzenoid compounds rarely undergo DA reactions and often require very reactive dienophiles. One example of such rare reaction is the Wagner-Jauregg reaction.
The dienophile
In a typical Diels-Alder reaction, the dienophile has an electron-withdrawing group conjugated to the alkene. Though common, this feature is not exclusive of Diels-Alder dienophiles. There must be some extra conjugation, at least a phenyl group or chlorine atom. The dienophile can be activated by a Lewis acid such as niobium pentachloride.[10]
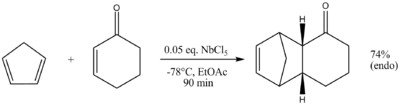
Cyclopentadiene does not react with cyclohexenone in ethyl acetate unless the Lewis acid is present. The yield improves when reaction temperature is lowered to -78°C because polymerization side reactions are prevented. Niobium pentachloride catalysis gives only the endo conformer. The same reaction with aluminium chloride results in an endo and exo mixture. Many of these Lewis acids are not good catalysts for the reaction of α,β-unsaturated carbonyls, this is because the carbonyl oxygen binds too tightly to the metal centre. A far better catalyst for such a system is a combination of silver perchlorate and Lawesson's reagent in cold dichloromethane.
It is well known that it is possible to use heteroatom containing dienophiles for Diels-Alder reactions, for instance Lawesson's reagent (and diferrocenyl dithiadiphosphetane disulfide) can react with 1,3-dienes to form six membered ring adducts. Also selenoketones and thioketones are able to react in the same way with 1,3-dienes. Imines are reactants in the Aza Diels-Alder reaction and carbonyl groups the reactant in Oxo Diels Alder reactions. These reactions are collectively called hetero Diels-Alder reactions.
Dienophiles can be chosen to contain a "masked functionality". The dienophile undergoes Diels-Alder reaction with a diene introducing such a functionality onto the product molecule. A series of reactions then follow to transform the functionality into a desirable group. The end product cannot not be made in a single DA step because equivalent dienophile is either unreactive or inaccessible. An example of such approach is the use of α-chloroacrylonitrile (CH2=CClCN). When reacted with a diene, this dienophile will introduce alpha-chloronitrile functionality onto the product molecule. This is a "masked functionality" which can be then hydrolyzed to form a ketone. α-Chloroacrylonitrile dienophile is an equivalent of ketene dienophile (CH2=C=O), which would produce same product in one DA step. The problem is that ketene itself cannot be used in Diels-Alder reactions because it reacts with dienes in unwanted manner (by [2+2] cycloaddition), and therefore "masked functionality" approach has to be used.[11]
Other such functionalities are phosphonium substituents (yielding exocyclic double bonds after Wittig reaction), various sulfoxide and sulfonyl functionalities (both are acetylene equivalents), and nitro groups (ketene equivalents).
Heterodienophiles
No major loss in reactivity of dienophile is seen when one, or both, of the carbons are substituted for another variety of atom.[12] Carbonyl groups, for example, can successfully react with dienes to yield pyranoid rings. Generally, the endo transition state is favored in this case.
Nitroso compounds (N=O) react to form oxazine-like compounds (cyclic molecules with nitrogen and oxygen present in the six-membered ring).[13] Another group of dienophiles successfully used for Diels-Alder reactions is imines.[14] Such reactions are useful for preparation of alkaloid and other polycyclic compounds.
Stereoselectivity in DA Reactions
Diels-Alder reactions can lead to formation of a variety of structural isomers and stereoisomers (enantiomers and diastereomers).[15] Identity of major products can usually be predicted, however.
In unsymmetrically substituted diene and dienophile, pseudo-ortho and para orientations in products are usually favored over meta orientation. A particular preference in location of substitutents in the product can, in some cases, be explained in terms of frontier orbital theory. Most commonly, diene bears an electron-releasing group (ERG) and dienophile bears an electron-withdrawing group (EWG). The strongest interaction takes place between HOMO of diene and LUMO of dienophile. Carbons that have the highest coefficients in two frontier orbitals will begin to bond; therefore these carbons will direct the orientation of substituents and thus identity of major product of a DA reaction.
Dealing with the actual frontier orbital coefficients can be avoided since the preferred orientation in product can be described in terms of partial positive and negative charges that exist in diene and dienophile. Carbon with a partial negative charge will interact most readily with carbon bearing a partial positive charge. Therefore those two carbons will start coming together, thus dictating the relative orientation of substituents. The existence of partial positive/negative charge can always be determined by drawing resonance contributors for diene and dienophile, taking their ERG and EWG into consideration.
The initial potential of the reaction was utilized in the form of insecticides, which ultimately led to the endo rule. Otto Diels’ and Kurt Alder’s reaction allowed for the production of weapons against agricultural pests to be fully realized in the 1930’s [16]. Most of these chemicals contained chlorine, of which two are called Dieldrin and Aldrin after the appropriate individuals. These chemicals have been long-since discontinued because of their toxicity to not only invertebrates, but to higher orders of organisms as well [17][18]. Fortunately, insecticide use may continue unabated with the recent introduction of various safe treatments. Though insecticides like Dieldrin and Aldrin cause a slew of cardiac and respiratory illnesses (as well as reproductive failure), they served as an important step towards understanding why the endo product was the major yield. The study of the insecticide’s fused norcamphane rings became a highly popular topic in the 1930s and 1940s. Oxidative degradation revealed high specificity of the stereochemistry; after much research by Kurt Alder and H.F. Rickert, it became clear that steric hindrance is not as important in Diels-Alder reactions as it is in other reactions. This led to the secondary orbital explanation as well as a satisfactory hypothesis that elucidated polymerization of certain Diels-Alder adducts [16].

Cis principle
According to the cis principle or the Alder-Stein rules formulated by Alder and Stein in 1937, the stereochemistry of substituents in the starting material is retained in the product. This means that if a cis-dienophile is reacted, both of the cis-substituents will end up on same side (face) of the product ring. Trans-dienophile will yield a product where both of trans-substituents (that came originally from the dienophile) will be on different sides of the product ring. The same principle applies to dienes. Trans, trans or cis, cis 1,4-substituents will end up on same side of the ring, whereas trans, cis 1,4-substituents will be oriented towards different faces of the ring.
Besides the ortho/meta/para-forming orientations, the diene and dienophile may arrange themselves in different ways to yield exo and endo transition states which result in different products. To determine which is the endo and which is the exo transition state, the two molecules are oriented parallel to each other, such that diene's single bond (one which connects two double bonds) is parallel to dienophile's double or triple bond. It makes no difference whether the dienophile is positioned above or below the diene. The single substituent (or cis-substituents on the dienophile) is oriented to point in the direction of diene's pi-system. This is the endo transition state (pictured below). If these substituents are pointed away from the diene, this would be the exo transition state.
Endo addition rule
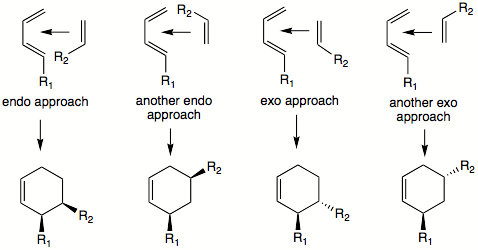
Using the 'cis principle' it is understood that cis-substituents on dienophile, for example, will end up on same side of the molecule. It is not obvious where the substituents on both diene and dienophile will end up relative to each other. To predict the cis or trans orientation of substituents that are coming from different molecules we have to examine possible transition states. The most stable transition state will lead to the major product. Transition states will also dictate the relative orientation of the diene's and dienophile's substituents on the product ring. In some cases another rule can be applied: the endo addition rule. According to this rule, the most stable transition state results when there is a 'maximum accumulation of double bonds'. This rule is not always followed. It most often applies when dealing with cyclic dienes and dienophiles. For example, the DA reaction of cyclopentadiene and maleic anhydride yields over 95% of the endo product. It is important to note that labels "exo" and "endo" relate to the orientation of substituents in the transition state and not to a specific orientation of substituents in the product molecule. In each individual case, the transition state has to be examined to see the most favored relative orientation of substituents. It is not true for the endo transition state that the substituents on dienophile and 1,4-substituents on diene will always point towards the same side of the newly formed ring. "Endo" and "exo" define specific transition states, not orientation of substituents. In the picture below, it just happens that the endo transition state will yield substituents on same side of the ring. This is not always so. In the case of maleic anhydride and cyclopentadiene the endo product will have the R groups of the diene and dienophile oriented toward the opposite sides of the newly formed ring.

The exo product can predominate, however, for some reactions. This can happen when the resulting endo product can easily dissociate back into the starting material. In such reactions, the exo product predominates with extended reaction times because the exo product is thermodynamically favored. In other cases, the endo product can convert to what would be the exo product of the reaction. In the example below, endo product B was the only one isolated after the Diels-Alder reaction. However, letting the reaction go for prolonged periods of time also yielded substantial amounts of exo product A. The authors speculated that endo product B can epimerize to exo product A in the following way:

In summary, diastereoselectivity is based on the postulation of the transition state. For any given DA reaction, one can imagine one possible transition state being favored over the other due to steric, stereoelectronic, and complexing factors. Thus, predictions can be made on the identity of major product of a particular DA reaction by looking at the starting material available.
Retro Diels-Alder reactions
DA reactions are reversible and in a retro Diels-Alder reaction the diene and alkene are reformed. One representative reaction is the Rickert-Alder reaction[19] in which, thanks to favorable rearomatization, the oxidized cycloadduct of quinone and 1,3-cyclohexadiene at elevated temperatures eliminates ethylene to form anthraquinone.

Asymmetric DA reactions
In asymmetric Diels-Alder reactions only one of two possible enantiomers is formed. Asymmetric catalysis by organocatalysis is possible with catalysts based on an imidazoline skeleton (the MacMillan catalyst[20]) for instance in the reaction of cyclohexadiene with acrolein:[21]
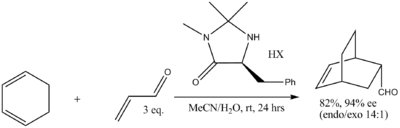
Diels-Alder reactions also lend themselves to chiral synthesis with chiral auxiliaries. In one research effort,[22] the auxiliary is derived from L-asparagine. The telescopic synthesis with cyclopentadiene and acrylic acid yields the DA adduct with three stereocenters as predominantly the endo conformer and with 54% ee.

Lewis acids (AlCl3, ZnCl2, and others) act as catalysts by coordinating to the dienophile. The complexed dienophile becomes more electrophilic and more reactive toward the diene. This increases the rate and often the stereoselectivity of a DA reaction.
References
- ↑ doi:10.1002/jlac.19284600106
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Synthesis of the hydro aromatic sequence, Ann. 1929, 470, 62.
- ↑ Synthesis in the hydroaromatic series, IV. Announcement: The rearrangement of malein acid anhydride on arylated diene, triene and fulvene, Diels, O.; Alder, K. Ber. 1929, 62, 2081 & 2087.
- ↑ Kloetzel, M. C. Org. React. 1948, 4, 1-59. (Review)
- ↑ Holmes, H. L. Org. React. 1948, 4, 60-173. (Review)
- ↑ doi:10.1021/cr00013a013
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ The Diels-Alder Reaction in Total Synthesis K. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis Angew. Chem. Int. Ed. 2002, 41, 1668-1698. (Review) doi:10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z
- ↑ Kozmin, S. A.; He, S.; Rawal, V. H. Organic Syntheses, Coll. Vol. 10, p.442 (2004); Vol. 78, p.160 (2002). (Article)
- ↑ Hershberg, E. B.; Ruhoff, J. R. Organic Syntheses, Coll. Vol. 2, p.102 (1943); Vol. 17, p.25 (1937). (Article)
- ↑ Niobium Pentachloride Activation of Enone Derivatives: Diels-Alder and Conjugate Addition Products Mauricio Gomes Constantino, Valdemar Lacerda Júnior and Gil Valdo José da Silva Molecules 2002, 7, 456–465. (Article)
- ↑ Ranganathan Synthesis 1977, 289.
- ↑ Weinreb, S. M. Comp. Org. Syn. 1991, 5, 513-550. (Review)
- ↑ Streith, J.; DeFoin, A. Synthesis 1994, 1107-1117.
- ↑ Greico, P.; Larsen, S. D. Organic Syntheses, Coll. Vol. 8, p.31 (1993); Vol. 68, p.206 (1990). (Article)
- ↑ Diastereofacial Selectivity in the Diels-Alder Reaction Coxon, J. M. et al. Advances in Detailed Reaction Mechanisms 1994, 3, 131-166. (Review)
- ↑ 16.0 16.1 Wasserman, A. Diels-Alder Reactions. New York, NY: Elsevier Company, 1965. pp. 1-3
- ↑ Damgaard, Ida N., Niels E. Skakkebaek, and et al. . "Persistent Pesticide in Human Breast Milk and Cryptorchidism." Environmental Health Perspectives 114 (2006): pp. 1133-1138.
- ↑ Cerrillo, I, M F. Olea-Serrano, J Ibarluzea, and et al. . "Environmental and Lifestyle Factors for Organochloride Exposure Among Women Living in Southern Spain." Chemosphere 62 (2006): pp. 1917-1924.
- ↑ Alder, K.; Stein, G.; Pries, P.; Winckler, H. Ber. Dtsch. Chem. Ges.1929, 62B, 2337-72.
- ↑ http://www.springerlink.com/content/h224043227h18428/
- ↑ doi:10.1021/ja000092s
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ A fully-telescoped, aqueous, auxiliary-mediated asymmetric transformation Mathew P. D. Mahindaratne, Brian A. Quiñones, Antonio Recio III, Eric A. Rodriguez, Frederick J. Lakner, and George R. Negrete Arkivoc(EJ-1566C) pp 321-328 2005. (Article)
External links
- Asymmetric Hetero-Diels-Alder Reactions
- Semi-empirical calculations of the Diels-Alder reaction.
- Endo Addition Rule